Process for the production of beta-lysine
A lysine and aspartokinase technology, applied in the field of recombinant microorganisms, can solve the problems of time-consuming and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
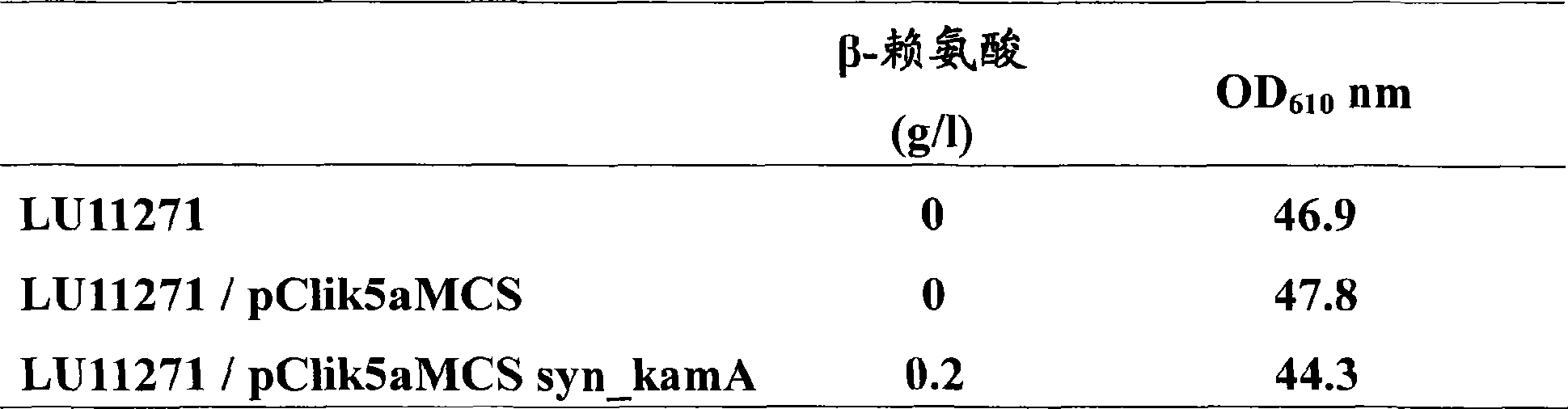
[0060] 1. Cloning of the proximal Clostridium lysine 2,3-aminomutase gene
[0061] According to the conserved regions upstream and downstream of the lysine 2,3-aminomutase genes of Fusobacterium nucleatum and Thermoanaerobacter tengcongensis, a set of oligonucleotide primers were designed for In the isolation of the proximal Clostridium lysine 2,3-aminomutase gene (kamA). Using PCR primers WKJ90 / WKJ65 and WKJ68 / WKJ93, and using the chromosome of Clostridium axeum as a template, the DNA fragments of the upstream and downstream regions, including the N-terminal and C-terminal sequences of the kamA gene, were amplified, respectively. After the sequence analysis of the amplified DNA fragment is completed, it is purified to obtain a product containing the start and end sequences of the kamA structural region. Based on the determined upstream and downstream sequences, PCR primers WKJ105 / WKJ106 were synthesized for isolating the full sequence of the Clostridium proximal kamA gene. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com