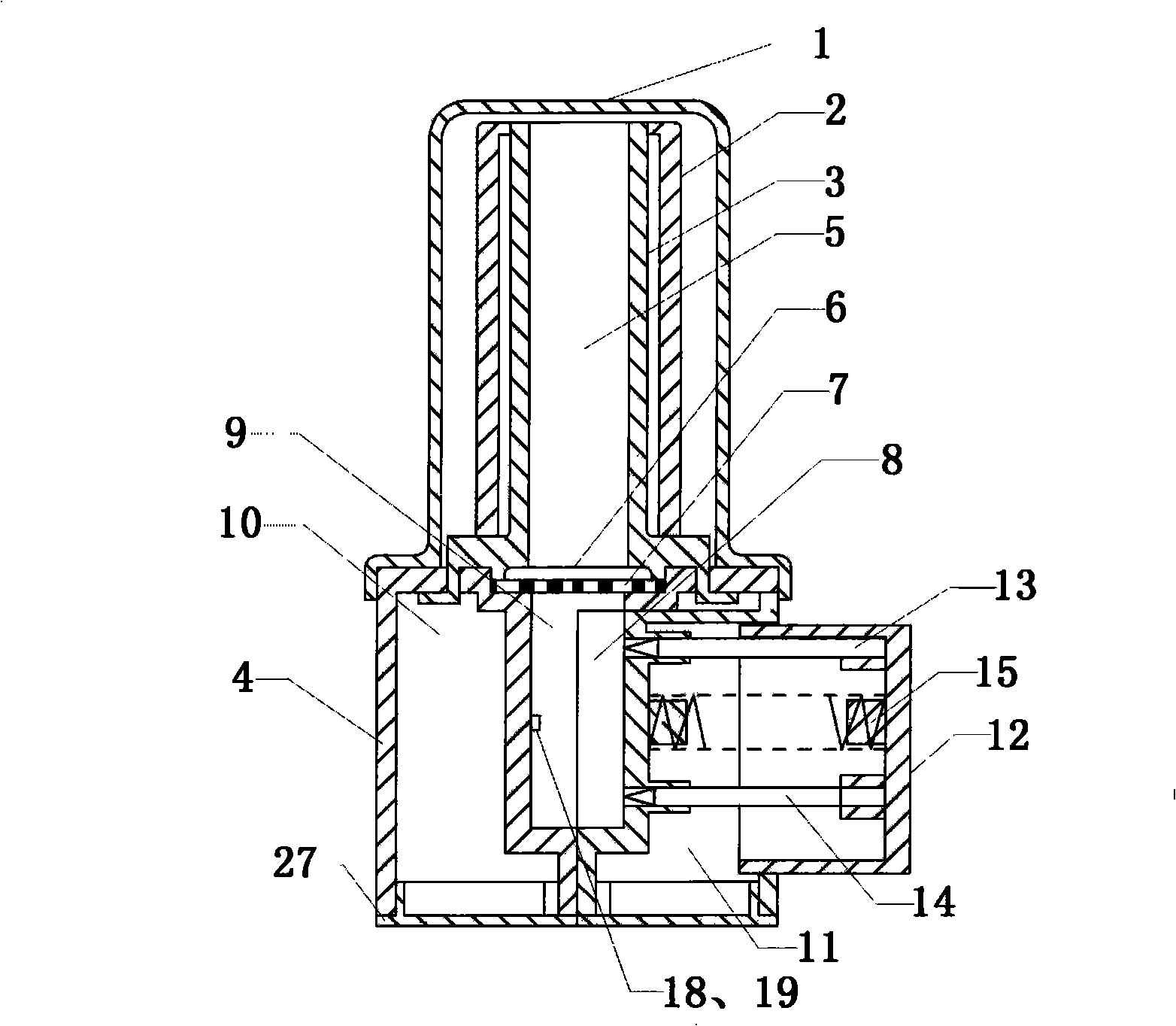
Capsule type inhalation dust cloud agent
A technology for inhaling powder aerosols and capsules, which is applied to the field of capsule-type inhalation powder aerosols, can solve the problems of less drug deposition, influence on curative effect, and high production cost, and achieve the effect of increasing deposition amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0074] The micronized active ingredient is tiotropium bromide with a particle size of 1.07 μm to 3.06 μm; the glycine carrier has a maximum particle size of 80 μm and an average particle size of 50 μm to 75 μm.
[0075] The fluidity angle of repose of the dry powder composition made of micronized tiotropium bromide and glycine carrier is 56°.
[0076] The hygroscopicity of the dry powder composition made of micronized tiotropium bromide and glycine carrier was 7.5% relative weight gain.
[0077] use The emptying rate of the dry powder inhalation device was 98.6%, and the drug deposition in the effective part was 20.6%.
[0078] The dry powder composition made of micronized tiotropium bromide and glycine carrier has no obvious irritation and allergic reaction.
[0079] The results of influencing factor test, accelerated test and long-term test show that the quality is stable.
example 2
[0081] The micronized active ingredient is tobramycin with a particle size of 2.01 μm to 3.54 μm; the glycine carrier has a maximum particle size of 135 μm and an average particle size of 70 μm to 105 μm.
[0082] The fluidity angle of repose of the dry powder composition made of micronized tobramycin and glycine carrier is 53°.
[0083] The hygroscopicity of the dry powder composition made of micronized tobramycin and glycine carrier was 5.5% relative weight gain.
[0084] use The emptying rate of the dry powder inhalation device was 96.8%, and the drug deposition in the effective part was 17.6%.
[0085] The dry powder composition made of micronized tobramycin and glycine carrier has no obvious irritation and allergic reaction.
[0086] The results of influencing factor test, accelerated test and long-term test show that the quality is stable.
example 3
[0088] The micronized active ingredient is ribavirin with a particle size of 1.21 μm to 2.14 μm; the glycine carrier has a maximum particle size of 115 μm and an average particle size of 50 μm to 95 μm.
[0089] The fluidity angle of repose of the dry powder composition made of micronized ribavirin and glycine carrier is 56°.
[0090] The hygroscopicity of the dry powder composition made of micronized ribavirin and glycine carrier was 5.4% relative weight gain.
[0091] use The emptying rate of the dry powder inhalation device was 97.9%, and the drug deposition in the effective part was 21.3%.
[0092] The dry powder composition made of micronized ribavirin and glycine carrier has no obvious irritation and allergic reaction.
[0093] The results of influencing factor test, accelerated test and long-term test show that the quality is stable.
[0094] 4. Example 4
[0095] The micronized active ingredient of the present invention is albuterol sulfate with a particle size of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Maximum granularity | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com