Anti-activated state T cell antibody vaccine for preventing and/or treating immune correlated disease
An immune-related disease, activated state technology, applied in the field of anti-activated T cell antibody vaccines, can solve problems such as induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1, the mechanism of action of the vaccine for preventing and / or treating immune-related diseases of the present invention
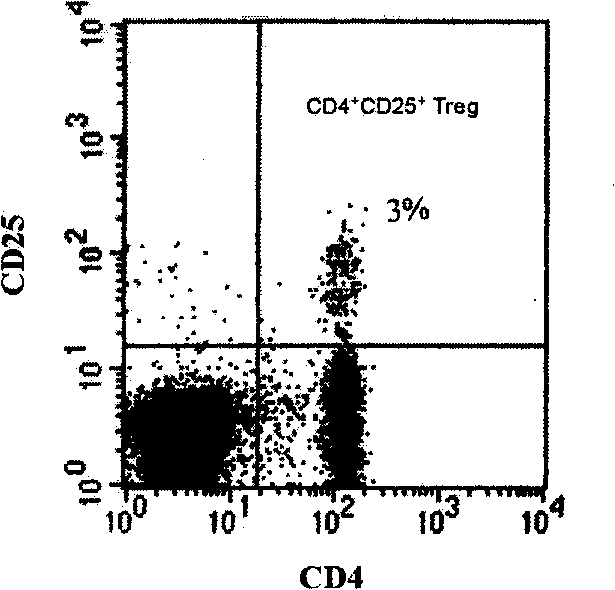
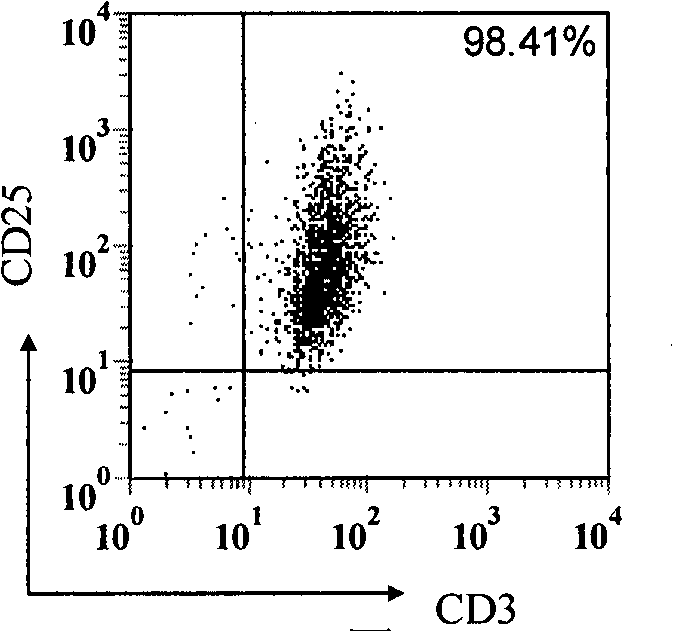
[0080] 1. The target proteins recognized by anti-activated T cell antibodies are calreticulin (CRT), ERp57 and vimentin
[0081] In the process of studying the mechanisms of TCV-induced immune regulation, people have gained a better understanding of TCV-induced regulatory T cell responses, but little is known about TCV-induced specific humoral responses (ie, antibodies). In the present invention, the ovalbumin (OVA)-specific T cell line derived from BALB / c mice was used as a vaccine to immunize mice of the same strain, and it was found that TCV could induce a humoral immune response against activated T cells. This result reveals the existence of anti-T cell regulatory antibodies, lays the foundation for future research on regulatory anti-T cell antibodies (regulatory anti-T-cell Abs), and also proposes a new approach for the treatment of...
Embodiment 2
[0153] Embodiment 2, the immune prevention and immunotherapy effect of vaccine of the present invention
[0154] 1. Treatment of CIA
[0155] CFA was used to induce arthritis (AIA) in Lews rats, and after three immunizations, adoptive transfer of OVA immunized rat serum (OVA) (with OVA as the immunogen, the antiserum obtained by immunizing Lews rats with conventional methods), vimentin immunized rat serum (antiserum obtained by immunizing Lews rats with full-length vimentin by conventional methods), ERp57-1 immunized rat serum (antiserum obtained by immunizing Lews rats by conventional methods with ERp57-1 as immunogen), calreticulin -1 Immune rat serum (with calreticulin-1 as the immunogen, the antiserum obtained by immunizing Lews rats by conventional methods), RTV-S rat serum (except that the immunized animals are Lews rats, the preparation method is the same as that of RTV-S BALB / c mouse serum) and ATV-S rat serum (except that the immunized animal is Lews rat, the prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com