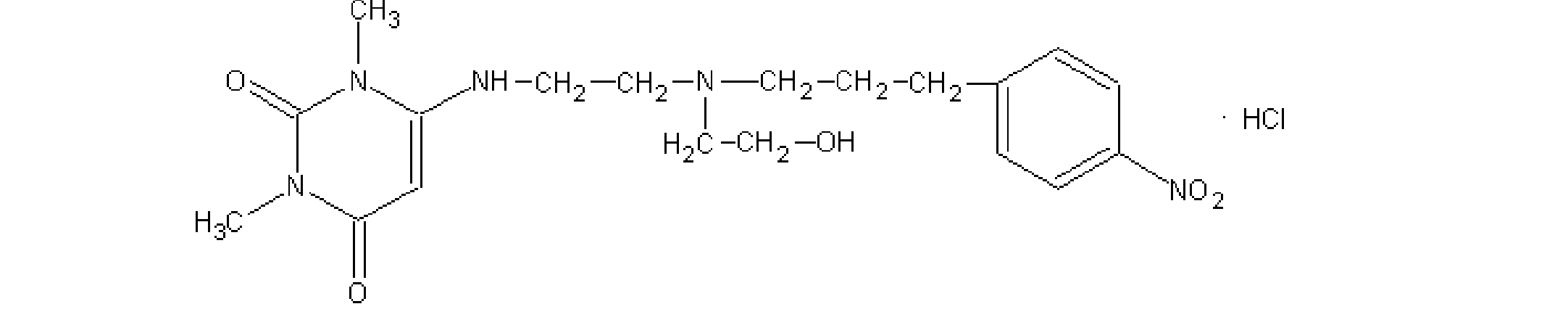Use of Nifekalant
A technology of nifecaran, uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In this example, nifekalant is used to prepare the following freeze-dried powder injections for the treatment of supraventricular arrhythmias such as atrial fibrillation and atrial flutter: the freeze-dried powder contains 50% nifekalant and 50% manna alcohol.
Embodiment 2
[0022] This example studies the acute effects of intravenous nifekalant on the patient's threshold for persistent and paroxysmal atrial fibrillation:
[0023] A total of 45 patients with sudden persistent atrial fibrillation were selected, and the duration of the onset of atrial fibrillation was greater than 24 hours. After the onset of atrial fibrillation, other antiarrhythmic drugs and direct current cardioversion were ineffective. The average age of the patients was 61.9±10 years old (43-78 years old), and the average ejection fraction was 56.4±13.8%. Among them, 4 cases suffered from mitral valve stenosis and 3 cases suffered from coronary heart disease. All patients had a history of taking other antiarrhythmic drugs before. The average duration of AF episodes in patients was 46.8±8.2 hours (35.8-49.5 hours). All patients received intracardiac defibrillation via an intravenous lead, but it was ineffective (failure to convert to sinus rhythm or immediate recurrence). The...
Embodiment 3
[0030]This example studies the enhancing effect of class III antiarrhythmic drugs (nifekalant) on the electrical defibrillation of hemodynamically deteriorated atrial fibrillation and the effect of preventing recurrence.
[0031] From the 1896 patients who entered the ICU ward due to cardiovascular disease, 201 patients with new onset atrial fibrillation were screened out, and 24 patients who met the following conditions were further screened out (the average age of the patients was 70±12 years old, and the systolic blood pressure was less than 90mmhg, resistant to general electrical defibrillation, 21 patients had congestive heart failure, 11 patients were mechanically ventilated, 19 were male, 5 were female) entered the clinical study: all patients were in the ICU Sinus rhythm, atrial fibrillation with recent hemodynamic deterioration, systolic blood pressure less than 90mmHg, QT interval less than or equal to 0.46 seconds, QRS wave group time greater than or equal to 0.14 se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com