Preparation of 2-(3-carboxaldehyde-4-hydroxy phenyl)-4-methyl-5-thiazole ethyl formate
A technology of ethyl thiazole formate and hydroxyphenyl is applied in the field of preparation of ethyl 2--4-methyl-5-thiazole formate, and can solve the problems of cumbersome post-processing, poor PPA fluidity, difficulty in mass production, and the like, Achieve the effect of increasing yield, reducing production costs and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
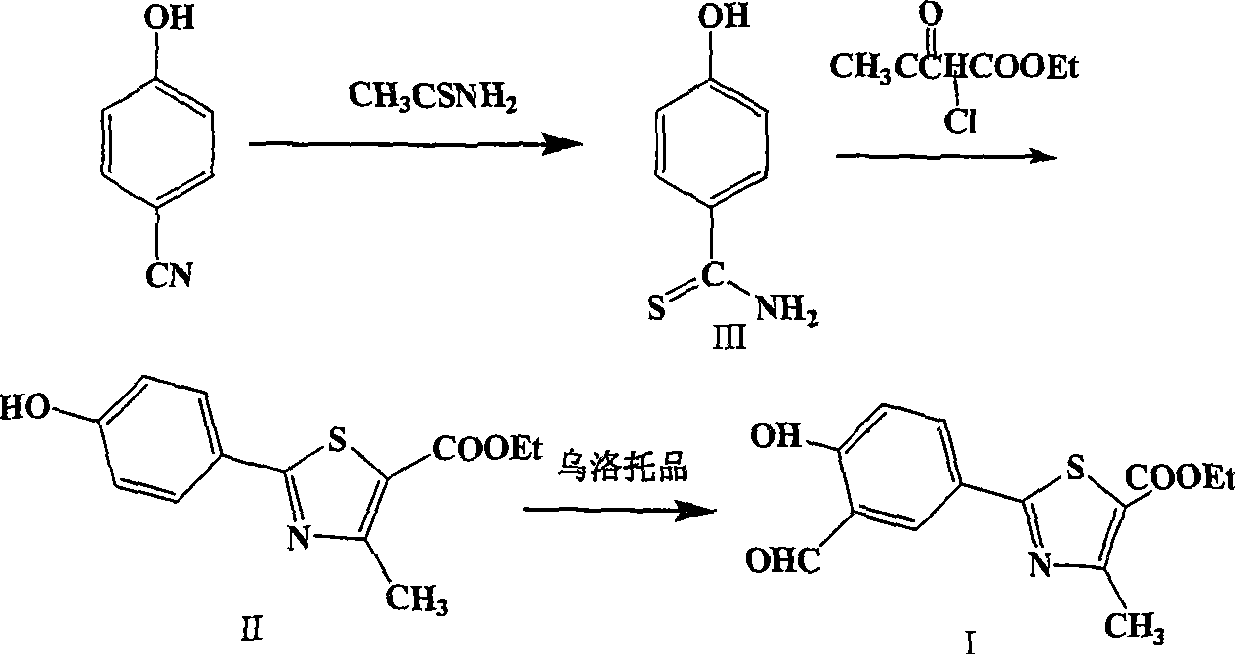
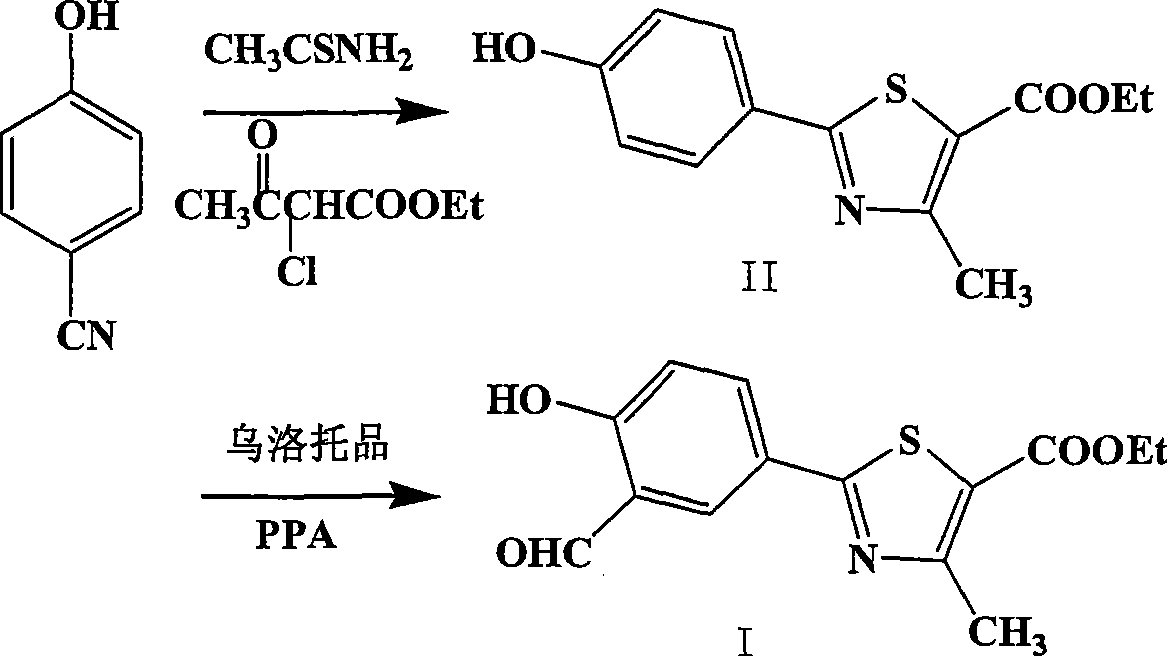
[0030] Embodiment 1: the preparation of 4-hydroxyl thiobenzamide (3)
[0031] In a 5L reaction flask, 400g of p-cyanophenol and 584g of thioacetamide were added to 4000ml of saturated HCl / DMF solution. Reacted at 40°C for 48 hours, TLC showed that the reaction was almost complete, concentrated to dryness, added saturated Na 2 CO 3 The solution was 1350ml, stirred for 2 hours, filtered, and dried to obtain 465.8g of brown solid (3). Yield 87.6%.
Embodiment 2
[0032] Embodiment 2: Preparation of ethyl 2-(4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate (2)
[0033] Add 465.8g of 4-hydroxythiobenzamide (3) and 2.8L of absolute ethanol into a 5L reaction flask, heat to 60°C, add 560g of ethyl 2-chloroacetoacetate dropwise, and then reflux for 2 hours. Then it was cooled to 10°C under stirring, filtered, washed with ethanol, and dried to obtain 721.1 g of yellow solid (2), with a yield of 80.6%.
Embodiment 3
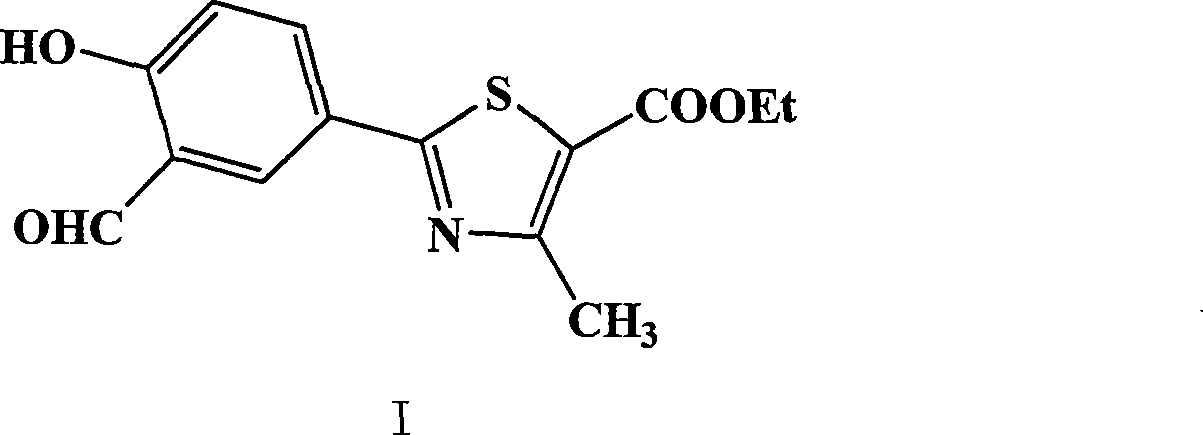
[0034] Example 3: Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate (1)
[0035] Add 120g of (2), 63.8g of urotropine and 660ml of trifluoroacetic acid into a 3L reaction flask, heat to 100°C for 24 hours, then concentrate the reaction solution to dryness, add 2L of water and stir for 5 hours, filter and dry to obtain 126.5 g of yellow solid (1), yield 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com