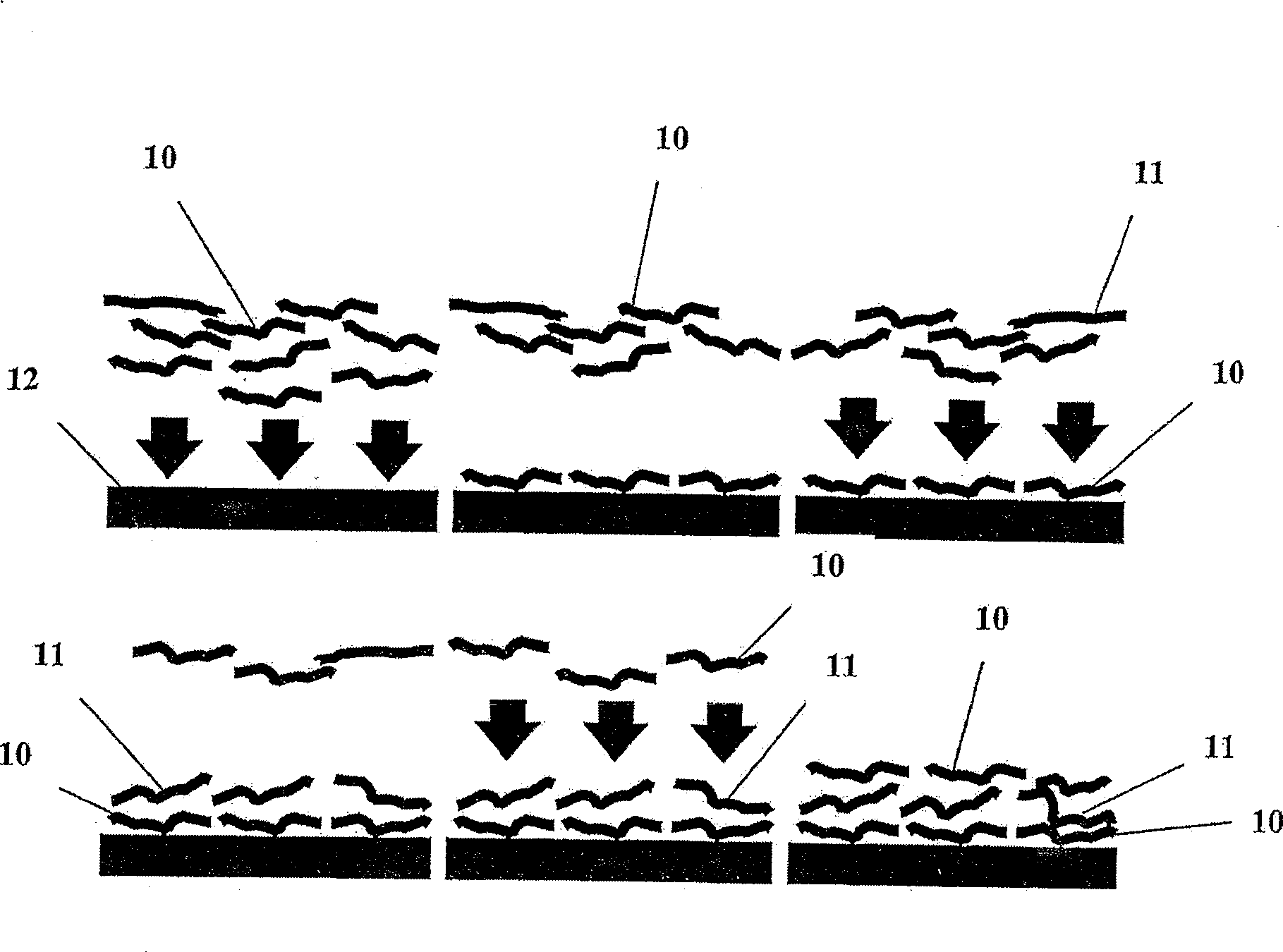
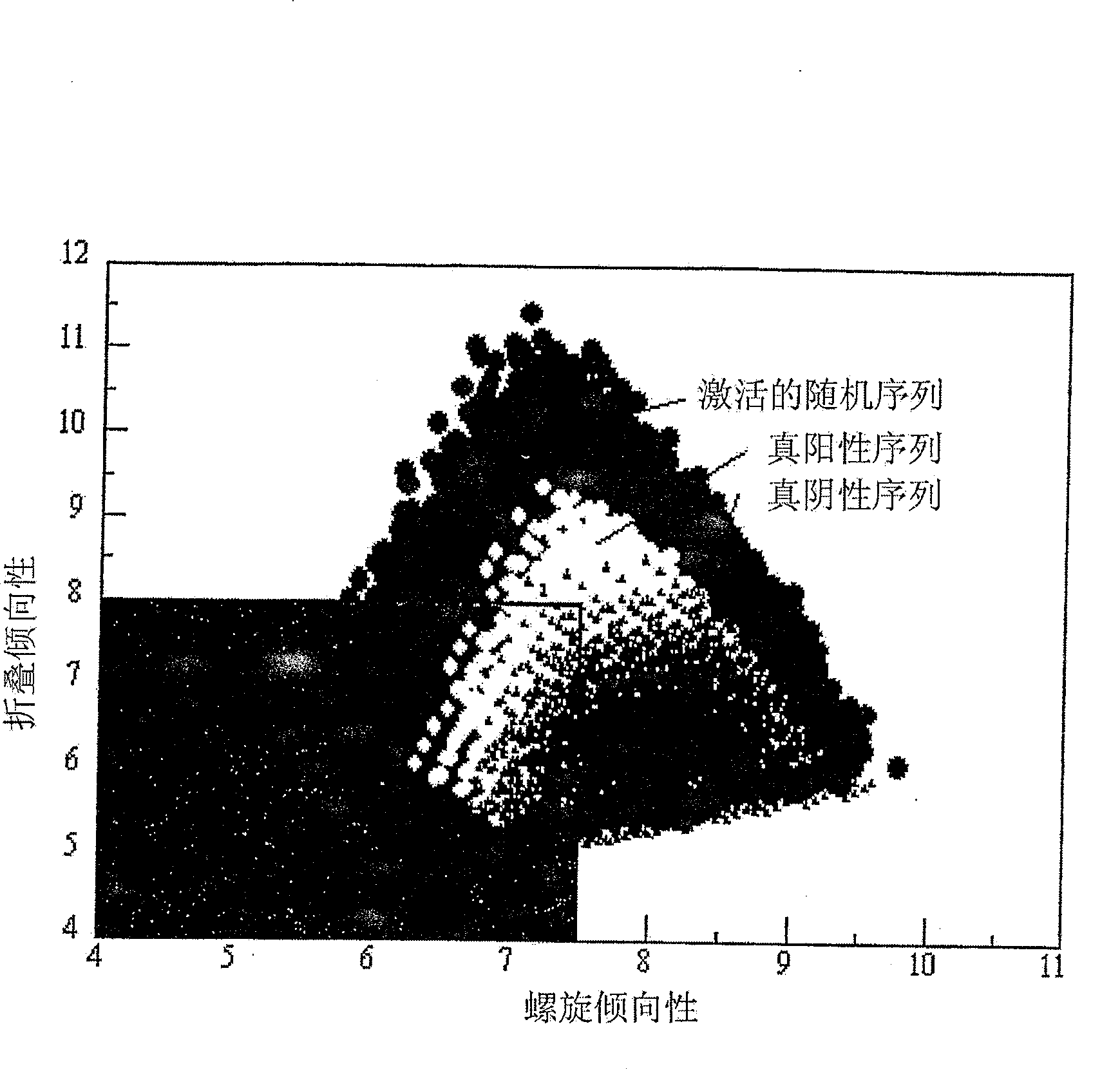
Method for designing polypeptides for the nanofabrication of thin films, coatings and microcapsules by electrostatic layer-by-layer self assembly
A self-assembling, thin-film technology for applications in biomedicine and other fields that can address issues such as lack of biocompatibility, scaling costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0135] 2. Example 2—Experiments Involving Redesigned Cysteine-Containing Polypeptides
[0136] a. Peptide
[0137] The peptides used are:
[0138] Tyr Lys Cys Lys Gly Lys Val Lys Val Lys Cys Lys Gly LysVal Lys Val Lys Cys Lys Gly Lys Val Lys Val Lys Cys Lys Gly Lys Val Lys (SEQ ID NO: 5)
[0139] Tyr Glu Cys Glu Gly Glu Val Glu Val Glu Cys Glu Gly GluVal Glu Val Glu Cys Glu Gly Glu Val Glu Val Glu Cys GluGly Glu Val Glu (SEQ ID NO: 6)
[0140] Unlike the other peptides used in the experiments described here, these two were not designed using human genome information; they were designed for the sole purpose of evaluating the role of disulfide bond formation in peptide film stabilization.
[0141] b. to operate
[0142] AU experiments were performed at room temperature. All assembly experiments using QCMs were performed under the same conditions, except that air was used instead of nitrogen to dry the samples that were about to undergo oxidation. Assembly conditions were 10 m...
example 3
[0151] 3. Example 3—Experiments Involving Cysteine-Containing Designer Polypeptides
[0152] a.Material
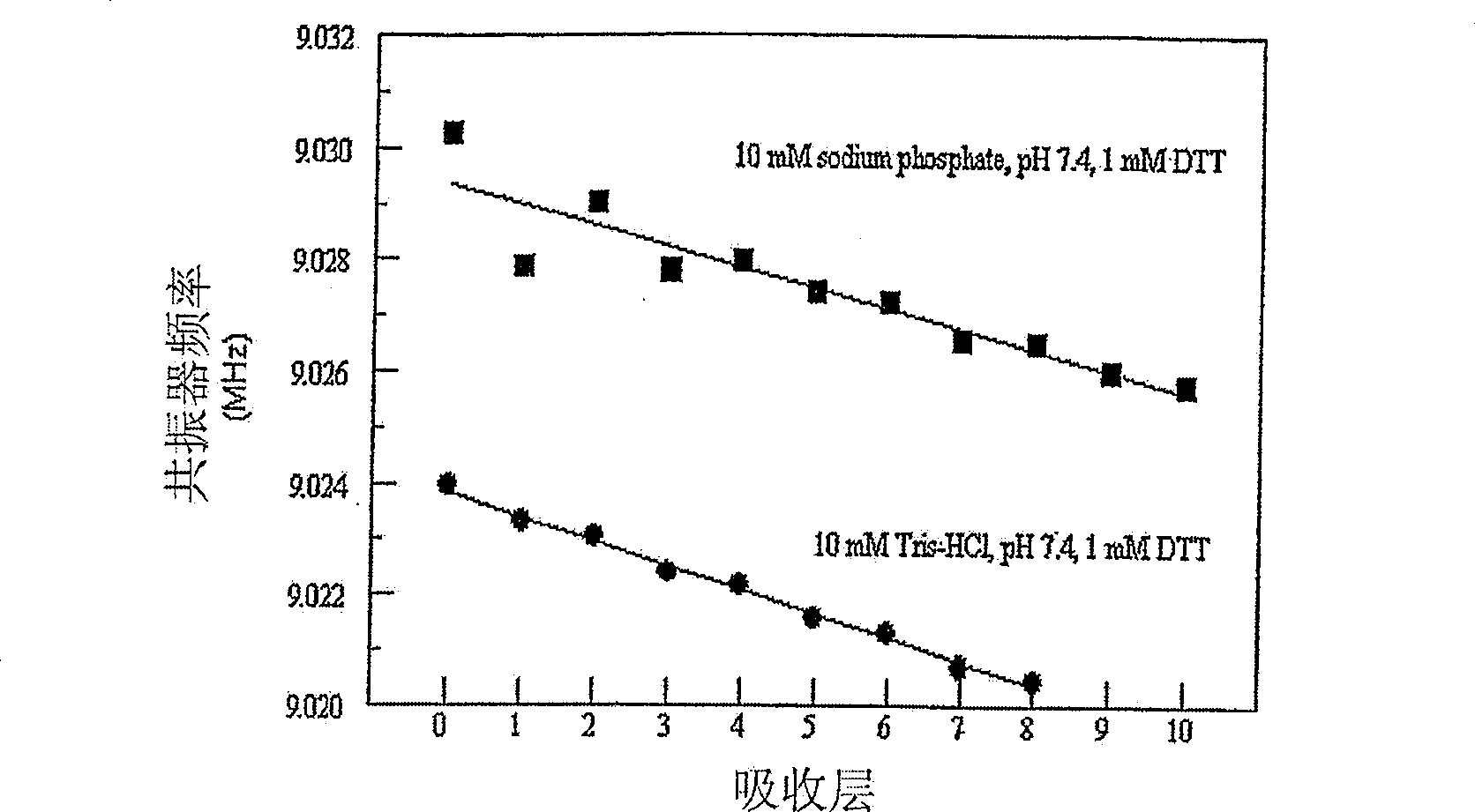
[0153] The essential elements of the experiment were a quartz crystal microbalance apparatus; a silver-coated resonator (9 MHz resonant frequency); a negative 48-residue peptide (LN3) (SEQ ID NO: 4); and the following sequence termed "SP5" The positive 48-residue peptide:
[0154] Tyr Lys Gly Lys Lys Ser Cys His Gly Lys Gly Lys Lys Ser Cys His Gly Lys Gly Lys Lys Ser Cys His Gly Lys Gly Lys Lys Ser Cys His (SEQ ID NO: 7)
[0155] Like the other designed peptides discussed in section VII(E)(1) above, SP5 was designed using the method described in VII(B)(1) above to analyze the amino acid sequence of the human blood protein lactoferrin (gi|4505043) . ELBL buffer was 10 mM Tris, pH 7.4, 10 mM NaCl and 1 mM DTT. The lysis buffer was 10 mM KCl, pH 2. 2 mL peptide solutions of SP5 and LN3 were prepared by adding 4 mg of each peptide to 2 mL of the above buffer solution and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com