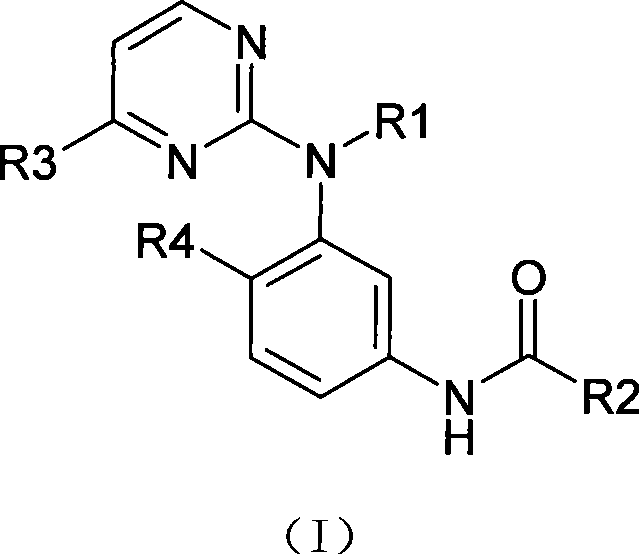
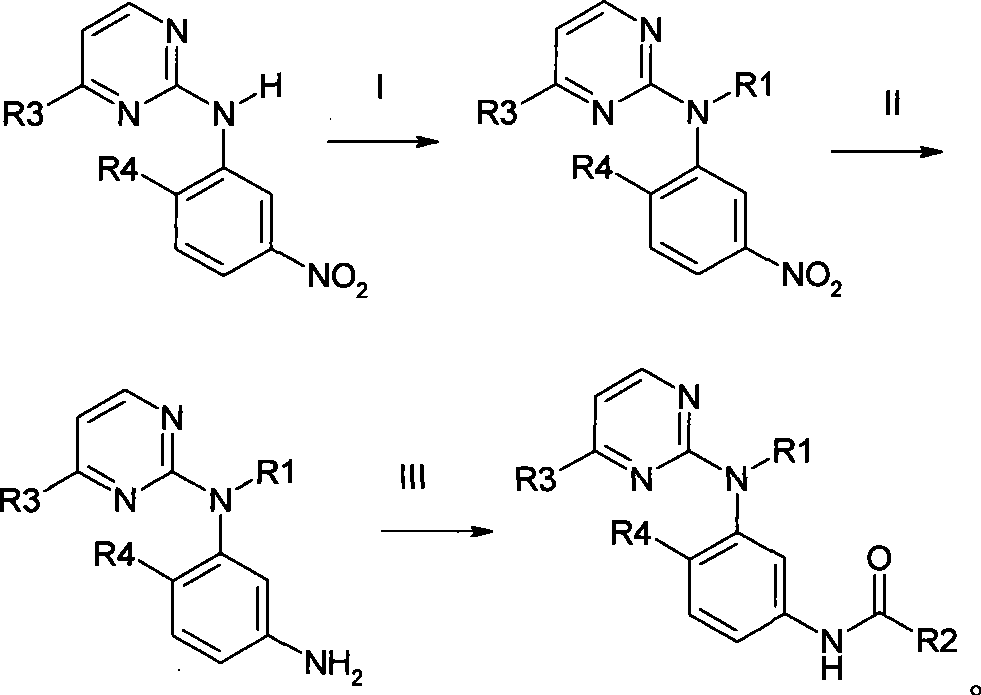
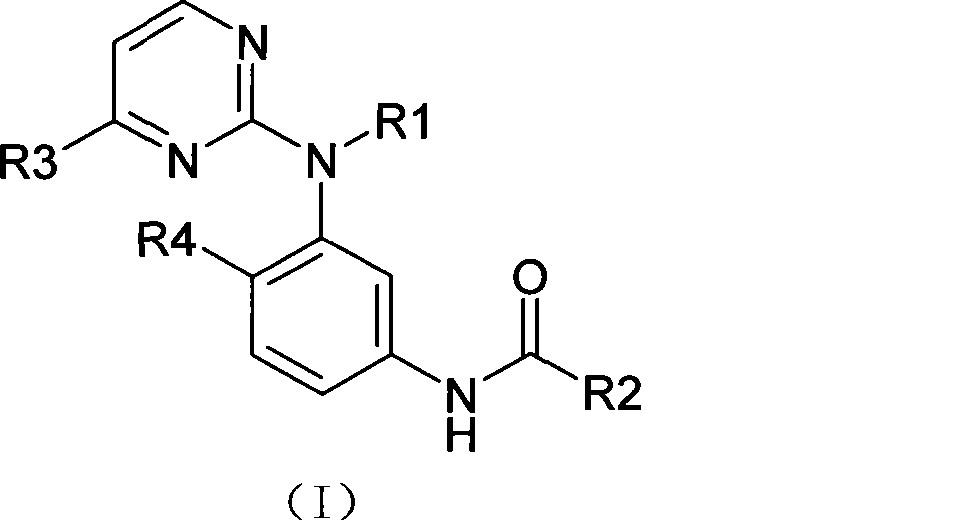
Amine pyrimidine derivates, and production method thereof, and medicament composition and use
A technology of anilinopyrimidine and pyrimidine, applied in the field of aminopyrimidine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1. N-(4-(pyridin-3-yl)pyrimidin-2-yl)-N-(2-methyl-5-nitrophenyl)acetamide
[0092]
[0093] Place 307mg of 4-(pyridin-3-yl)-2-(2-methyl-5-nitroanilino)pyrimidine in a 50ml flask, add 30ml of acetic anhydride and 100mg of anhydrous sodium acetate, and heat to 70oC After stirring for 5 hours, the acetic anhydride was removed under reduced pressure, 20 ml of water was added and extracted with ethyl acetate, washed with water, washed with a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate, and the solvent was removed to obtain N-(4-(pyridine-3 -yl)pyrimidin-2-yl)-N-(2-methyl-5-nitrophenyl)acetamide. 1 H NMR (300M, CDCl 3 ), δ (ppm) 9.12 (S, 1H, ArH), 8.65 (d, 1H, ArH), 8.62 (d, 1H, ArH), 8.17 (m, 1H, ArH), 8.10 (dd, 1H, ArH) , 8.02(d, 1H, ArH), 7.47(d, 1H, ArH), 7.43(d, 1H, ArH), 7.38(dd, 1H, ArH), 2.61(S, 3H, -COCH 3 ), 2.25 (S, 3H, -CH 3 ).FABMS: (M+H) + =350.
Embodiment 2
[0094] Example 2. N-(4-(pyridin-3-yl)pyrimidin-2-yl)-N-(2-methyl-5-aminophenyl)acetamide
[0095]
[0096] Place 349 mg of N-(4-(pyridin-3-yl)pyrimidin-2-yl)-N-(2-methyl-5-nitrophenyl)acetamide in a 100 ml flask, add 50 ml of methanol and 35 mg of 10% Pd / C, filtered after hydrogenation at normal pressure for 5 hours, and the solvent was spun off to obtain N-(4-(pyridin-3-yl)pyrimidin-2-yl)-N-(2-methyl-5 -aminophenyl)acetamide. 1 H NMR (300M, DMSO-d), δ (ppm) 9.27 (S, 1H, ArH), 8.82 (d, 1H, ArH), 8.72 (S, 1H, ArH), 8.46 (d, 1H, ArH), 7.97(d, 1H, ArH), 7.58(m, 1H, ArH), 6.95(d, 1H, ArH), 6.47(d, 1H, ArH), 6.39(dd, 1H, ArH), 2.32(S, 3H ,-COCH 3 ), 1.98 (S, 3H, -CH 3 ).FABMS: (M+H) + =320.
Embodiment 3
[0097] Example 3.N-[3-(acetyl-(4-pyridin-3-yl-pyrimidin-2-yl)amino)-4-methylphenyl]-3-acetamidobenzamide
[0098]
[0099] Dissolve 319 mg of N-(4-(pyridin-3-yl)pyrimidin-2-yl)-N-(2-methyl-5-aminophenyl)acetamide and 197 mg of 3-acetamidobenzoyl chloride in In 20 ml of anhydrous pyridine, stirred at room temperature for 12 hours, removed the solvent under reduced pressure, added ethyl acetate to dissolve, washed with saturated sodium bicarbonate solution, washed with water, washed with saturated brine, dried overnight with anhydrous sodium sulfate, spun to remove the solvent, and the column After chromatographic separation, N-[3-(acetyl-(4-pyridin-3-yl-pyrimidin-2-yl)amino)-4-methylphenyl]-3-acetamidobenzamide was obtained. 1 H NMR (300M, DMSO-D6), δ (ppm) 10.25 (S, 1H, N-H), 10.12 (S, 1H, N-H), 9.30 (d, 1H, ArH), 8.84 (d, 1H, ArH), 8.76(dd, 1H, ArH), 8.50(d, 1H, ArH), 8.02(m, 2H, ArH), 7.68(m, 2H, ArH), 7.59(m, 2H, ArH), 7.41(t, 1H , ArH), 7.32 (d, 1H, ArH), 7.80 (dd, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com