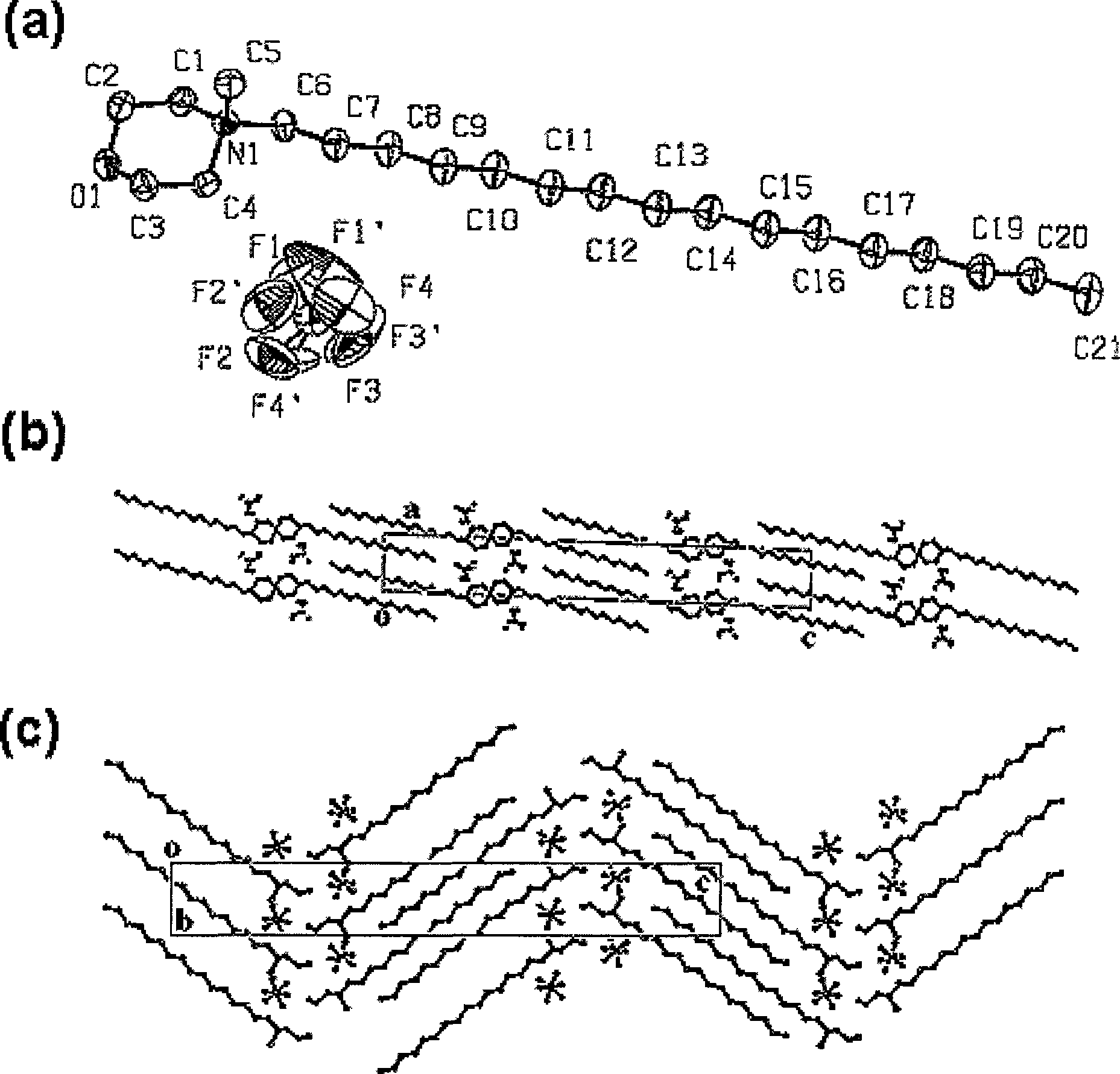
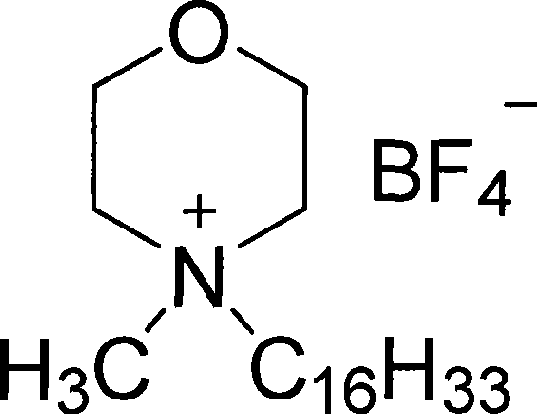
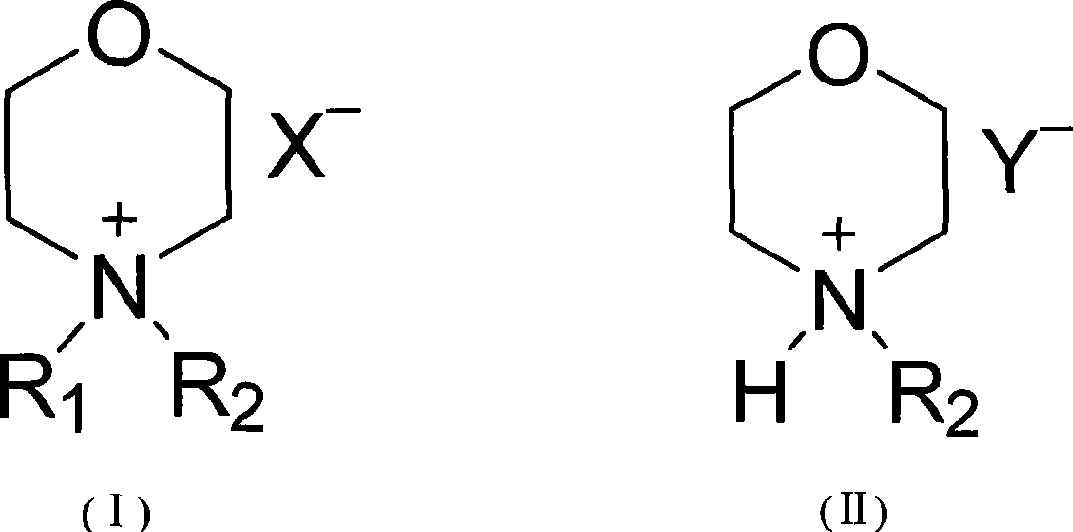Liquid crystal compound containing morpholine cation group, synthesis and uses thereof
A technology of liquid crystal compounds and ionic groups, used in chemical instruments and methods, liquid crystal materials, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of N-decyl-N-methyl-morpholine ammonium chloride
[0033] 10.12 grams (0.1mol) of N-methyl-morpholine, 176.1 grams (1mol) of chlorodecane and 150 grams of solvent ethanol were added to the reaction device, the reaction temperature was 150 ° C, and the pressure was 1 atmosphere and reacted for 100 hours. After the reaction was completed (chromatographic detection), the solvent was evaporated. The N-decyl-N-methyl-morpholine ammonium chloride compound can be obtained after the reaction product is recrystallized from acetonitrile, which is stable to air and easy to absorb water, with a yield of 94%. The liquid crystal temperature ranges from 42°C to 73°C.
[0034] 1 H-NMR (300MHz, D 2 O): δ=3.91(s, 4H), 3.38(m, 6H), 3.08(s, 3H), 1.67(s, 2H), 1.18(m, 14H), 0.73(m, 3H).Anal.Calcd .for C 15 h 32 CINO: C, 69.67; H, 12.25; N, 4.42%. Found: C, 69.99; H, 12.55; N, 4.36%. MS (ESI): m / z=242.3 (M + ).
Embodiment 2
[0036] Preparation of N-undecyl-N-ethyl-morpholine ammonium bromide
[0037] Add 11.51 grams (0.1mol) of N-ethyl-morpholine, 24.58 grams (0.105mol) of bromoundecane and 150 grams of solvent acetonitrile into the reaction device, the reaction temperature is 50 ° C, the pressure is 10 atmospheres, and the reaction time is After 10 hours, after the reaction was completed (chromatographic detection), the solvent was distilled off. The reaction product is recrystallized from acetonitrile to obtain N-undecyl-N-ethyl-morpholine ammonium bromide compound, which is stable to air and easy to absorb water, with a yield of 93%. The liquid crystal temperature ranges from 67°C to 86°C.
[0038] 1 H-NMR (300MHz, D 2 O): δ=3.81(m, 4H), 3.40(m, 6H), 3.28(m, 2H), 1.77(s, 2H), 1.18(m, 19H), 0.73(m, 3H).Anal.Calcd .for C 17 h 36 BrNO: C, 58.27; H, 10.36; N, 4.00%. Found: C, 58.10; H, 10.45; N, 4.16%. MS (ESI): m / z = 270.3 (M + ).
Embodiment 3
[0040] Preparation of N-tridecyl-N-ethyl-morpholine ammonium fluorophosphate
[0041] Add 11.51 grams (0.1mol) of N-ethyl-morpholine, 78.64 grams (0.3mol) of bromotridecane, and 200 grams of propanol into the reaction device. The reaction temperature is 100°C, the pressure is 5 atmospheres, and the reaction time is After 60 hours, after the reaction (chromatographic detection), 16.3 grams (0.1 mol) of ammonium fluorophosphate was added to the above reaction solution, and the reaction was carried out at 35°C for 50 hours, and the reaction product was recrystallized from acetonitrile to obtain N-tridecane Base-N-ethyl-morpholine ammonium fluorophosphate compound, stable to air and water, yield 96%. The liquid crystal temperature ranges from 73°C to 87°C.
[0042] 1 H-NMR (300MHz, CDCl 3 ): δ=3.84(m, 4H), 3.43(m, 6H), 3.28(m, 2H), 1.81(s, 2H), 1.22(m, 23H), 0.73(m, 3H).Anal.Calcd. for C 19 h 40 f 6 NOP: C, 51.46; H, 9.09; N, 3.16%. Found: C, 51.17; H, 9.45; N, 3.18%. MS (E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com