Method for producing unsaturated aldehyde and unsaturated carboxylic acid
A manufacturing method and unsaturated technology, which are applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of the reduction of the reaction rate of raw materials and the reduction of catalyst activity, and achieve the effect of long-term use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
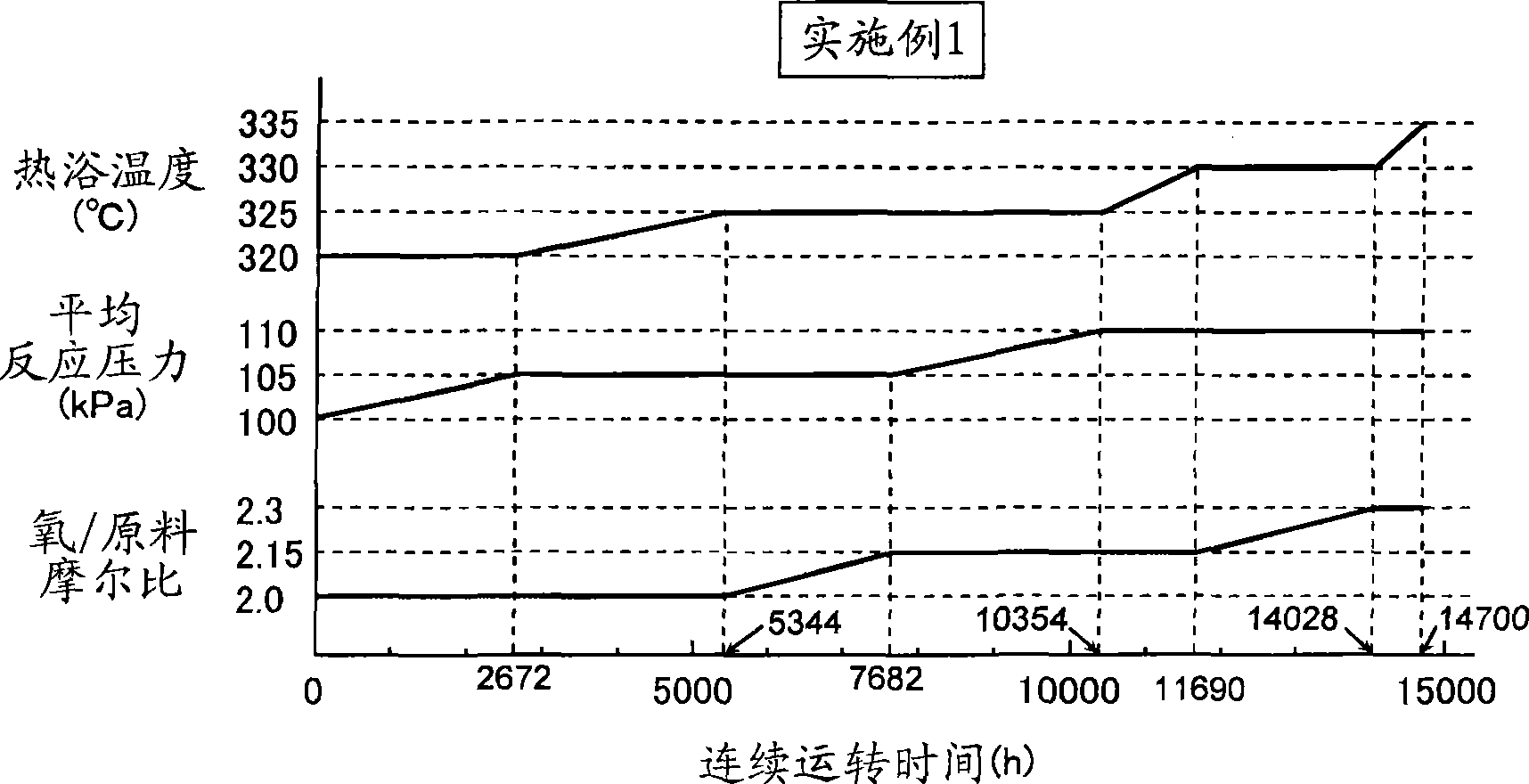
[0094] (1st response)
[0095] 2000 g of the catalyst obtained in Reference Example was filled in the reaction tube used in Reference Example. Next, the temperature of the heating bath was set at 320°C, and a reaction gas composed of 5 vol% of isobutylene (reaction raw material), 9 vol% of oxygen, 10 vol% of water vapor, and 76 vol% of nitrogen was passed through at a catalytic time of 4.5 seconds. Gas-phase catalytic oxidation of isobutene is carried out under the conditions of the catalyst layer. As a result of analyzing the initial reaction products, the reaction rate of isobutylene was 95.7%, the selectivity of methacrolein was 87.9%, and the selectivity of methacrylic acid was 5.4%. The temperature of the heat bath at this stage was 320° C., the average reaction pressure was 100 kPa, and the molar ratio of molecular oxygen in the reaction gas / raw material was 2.0 (initial reaction conditions).
[0096] (second response)
[0097] Next to the first reaction, control is p...
Embodiment 2
[0103] (1st response)
[0104] The first reaction was carried out in the same manner as in Example 1.
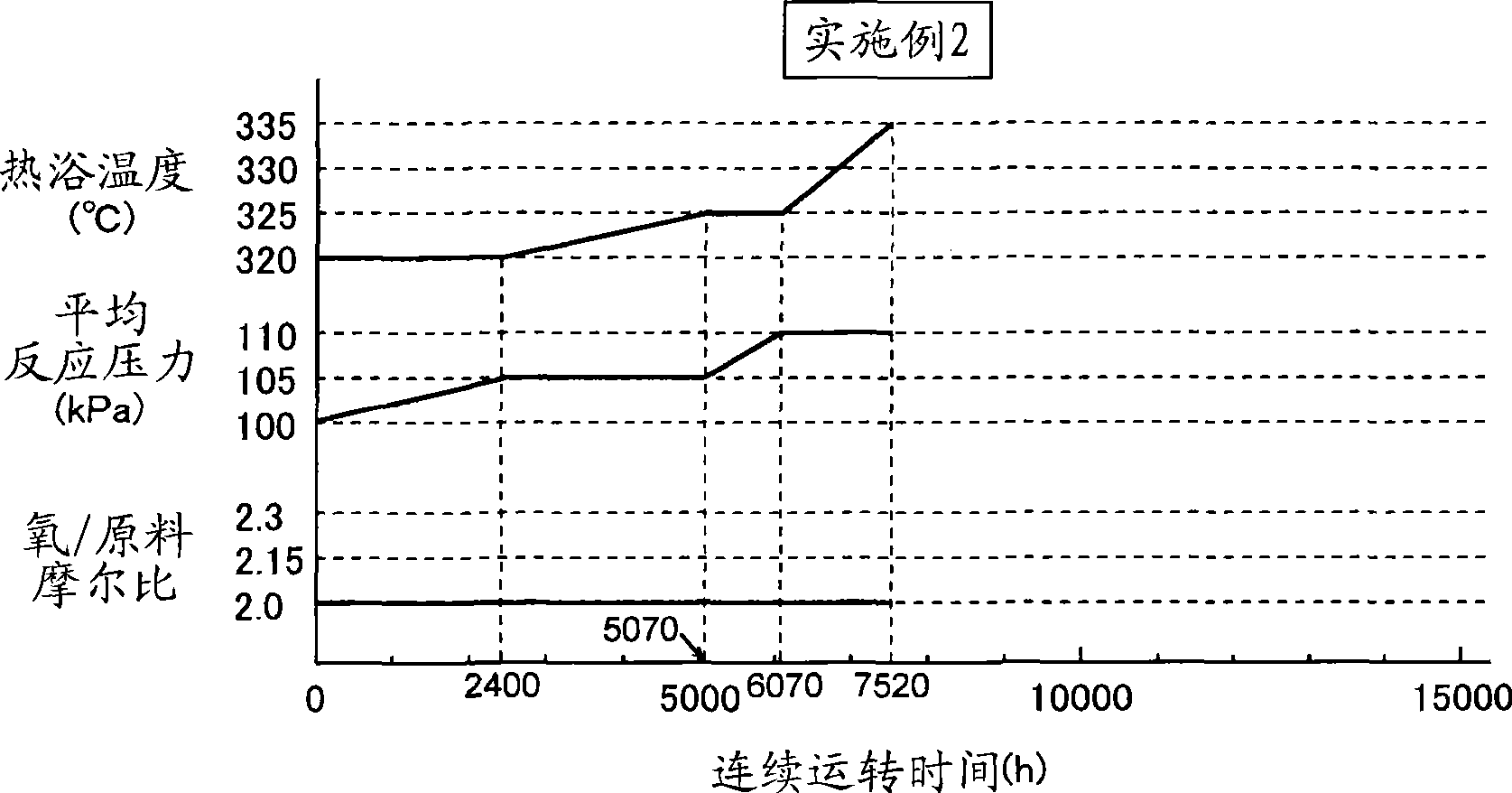
[0105] (second response)
[0106] In the 1st reaction, then, as figure 2 As shown, the second reaction was carried out in the same manner as in Example 1 except that the temperature of the heating bath and the average reaction pressure were changed. The cycle of continuous operation is 7520 hours. Analysis of the reaction product at this stage revealed that the reaction rate of isobutylene was 95.6%, the selectivity of methacrolein was 87.9%, and the selectivity of methacrylic acid was 5.4%. The temperature of the hot bath at this stage was 335° C., the average reaction pressure was 110 kPa, and the molar ratio of molecular oxygen / raw material in the reaction gas was 2.0 (reaction condition 2).
[0107] (3rd reaction)
[0108] After the second reaction, the third reaction was carried out in the same manner as in Example 1. Analysis of the initial reaction product at t...
Embodiment 3
[0112] (1st response)
[0113] The first reaction was carried out in the same manner as in Example 1.
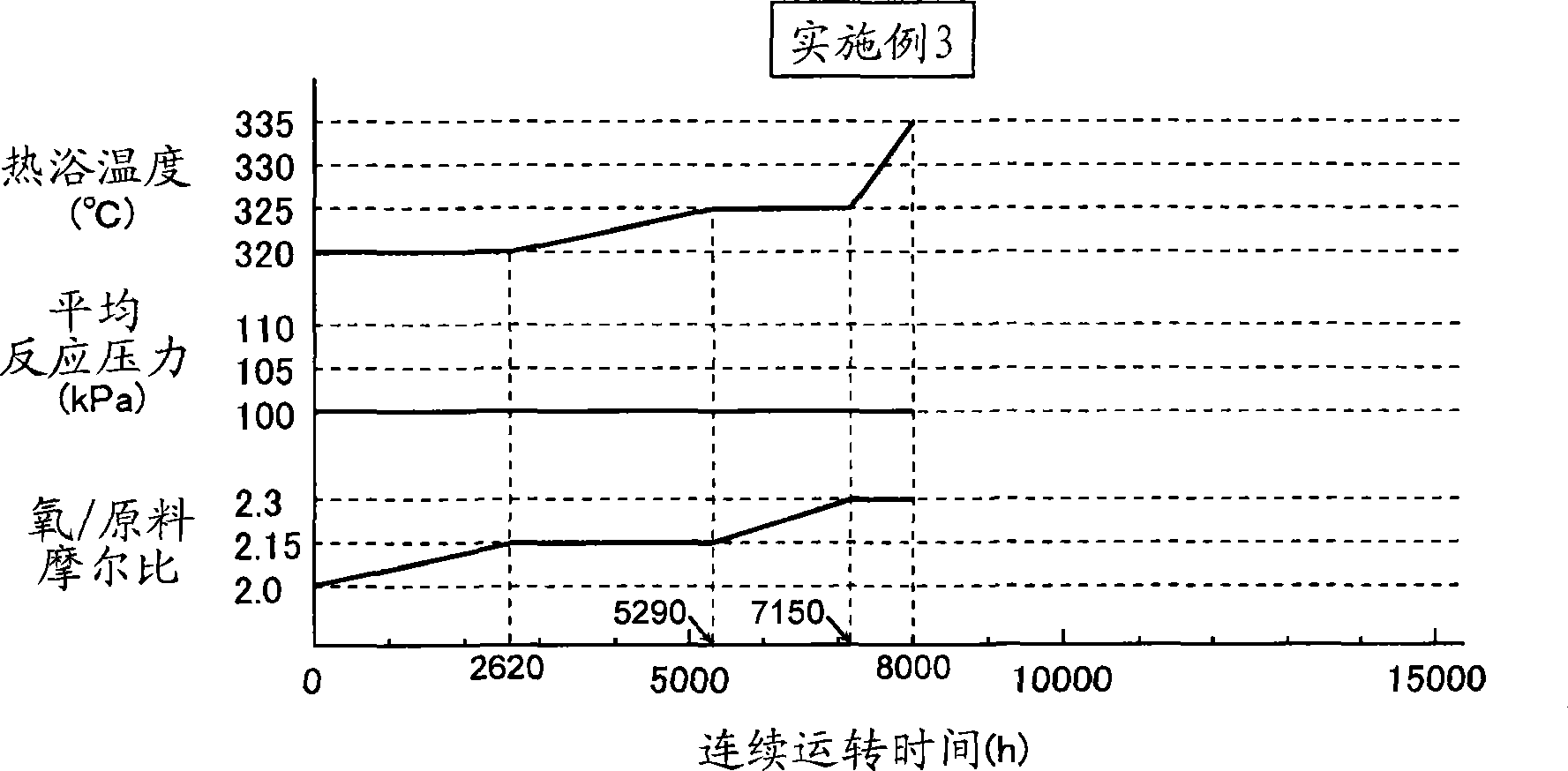
[0114] (second response)
[0115] Then the first reaction, such as image 3 As shown, the second reaction was carried out in the same manner as in Example 1, except that the temperature of the heating bath and the molar ratio of molecular oxygen in the reaction gas / raw material were changed. The cycle of continuous operation is 8000 hours. Analysis of the reaction products at this stage revealed that the reaction rate of isobutylene was 95.7%, the selectivity of methacrolein was 87.9%, and the selectivity of methacrylic acid was 5.4%. The temperature of the hot bath at this stage was 335° C., the average reaction pressure was 100 kPa, and the molar ratio of molecular oxygen in the reaction gas / raw material was 2.3 (reaction condition 3).
[0116] (3rd reaction)
[0117] After the second reaction, the third reaction was carried out in the same manner as in Example 1. An...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com