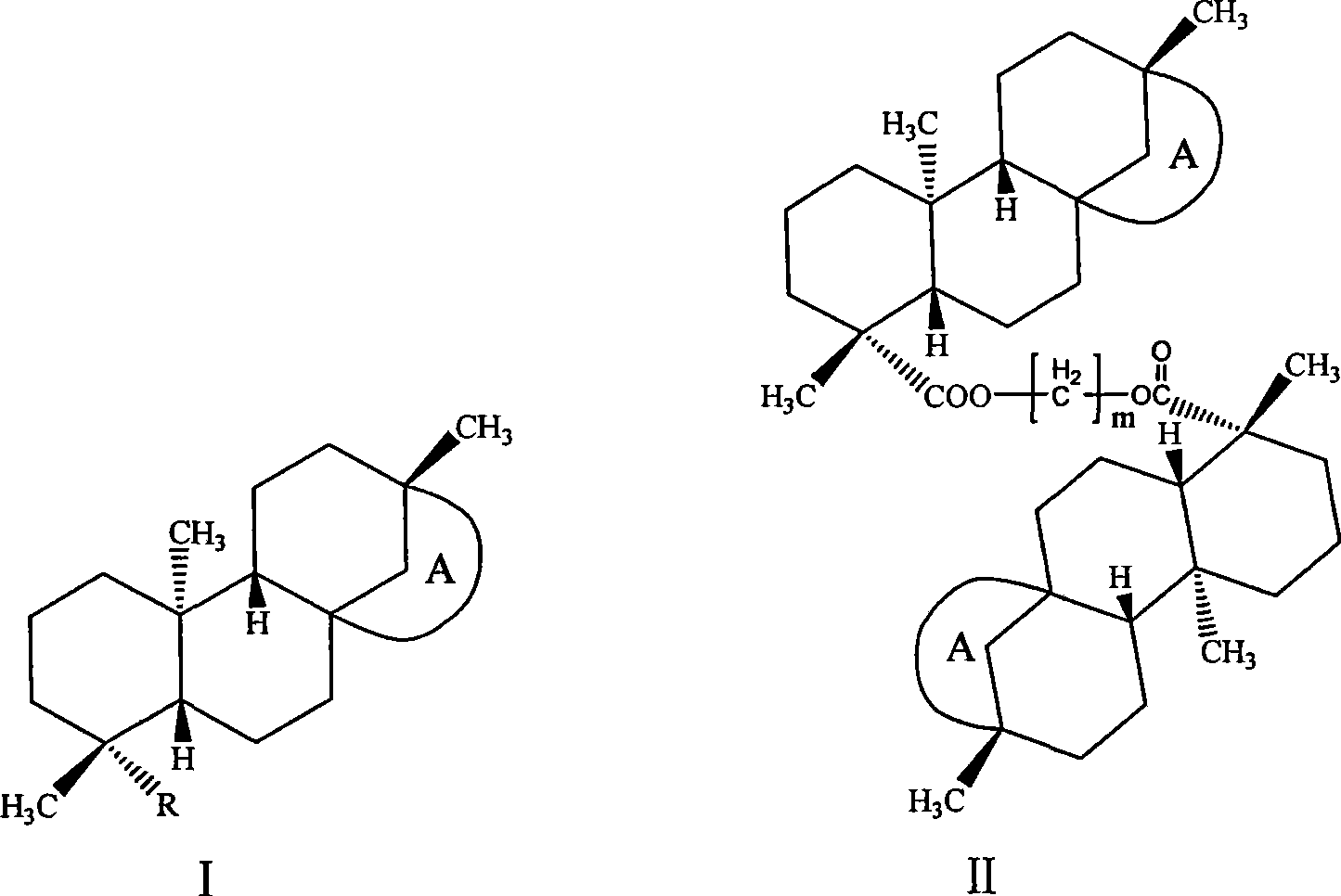
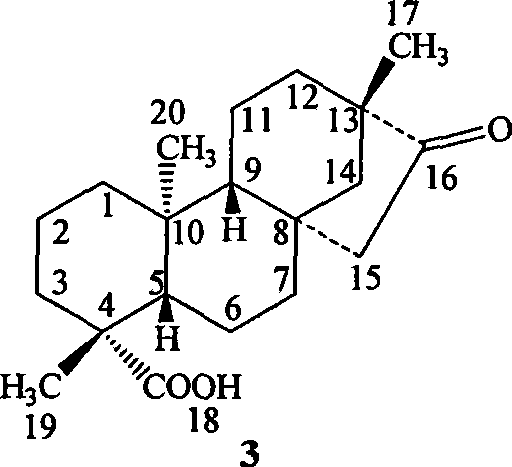
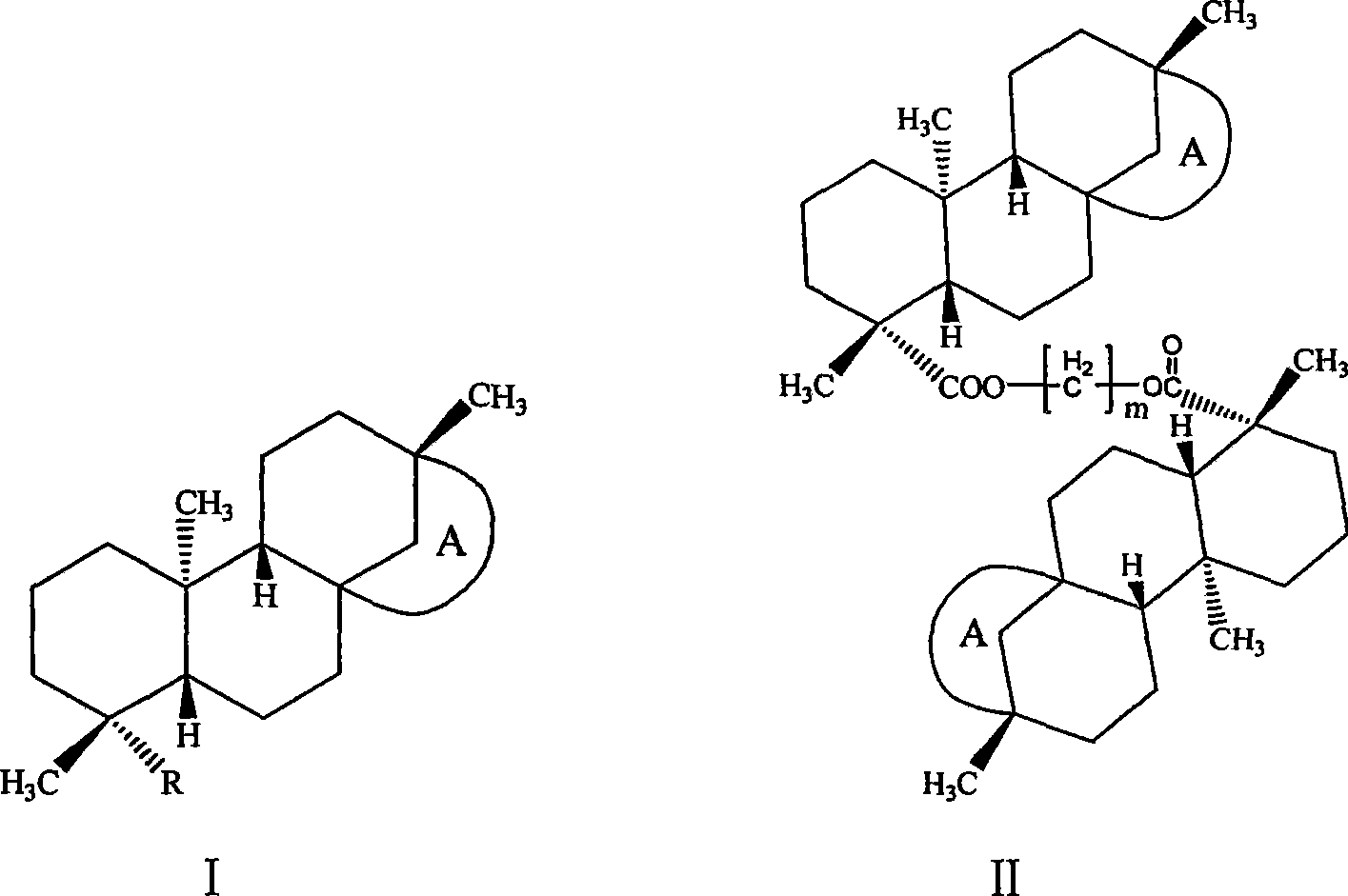
Isosteviol derivant and application thereof
A technology of isosteviol and its derivatives, which is applied in the direction of drug combination, active ingredients of heterocyclic compounds, medical preparations containing active ingredients, etc., and can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: (4α, 8β, 13β)-13-methyl-16-oxo-17-norkaurane-18-acid n-butyl ester (I 1 )
[0067] Dissolve 3g (0.0094mol) of isosteviol in 80mL of DMSO, add 6g of potassium hydroxide, 1.4mL (0.013mol) of 1-bromobutane, heat to reflux for 3h, pour into ice water, solids precipitate out, filter, filter The cake was recrystallized with ethanol to obtain 3.2 g of light yellow crystals, with a yield of 91%.
[0068] 1 H NMR (300MHz, CDCl 3 ),δ H , ppm: 0.71(s, 3H, 20-H 3 ), 0.98(s, 3H, 17-H 3 ), 1.19(s, 3H, 19-H 3 ), 0.91-1.88 (m, 25H, 1-H 2 ,2-H 2 , 3-H ax , 5-H, 6-H 2 ,7-H 2 , 9-H, 11-H 2 , 12-H 2 , 14-H 2 , 15-H β , COOCH 2 C 3 H 7 ), 2.16-2.21(d, 1H, 3-H eq , J=14.91Hz), 2.60-2.67 (dd, 1H, 15-H α , J=18.6, 3.69Hz), 3.98-4.10 (m, 2H, COOC H 2 C 3 h 7 )
Embodiment 2
[0069] Example 2: (4α, 8β, 13β)-13-methyl-16-oxo-17-norkaurane-18-acid isopropyl ester (I 2 )
[0070] In a 250mL three-necked flask, dissolve 5g (0.016mol) of isosteviol in 100mL of DMSO, add 10g of potassium hydroxide, 2mL (0.021mol) of 2-bromopropane, stir mechanically, heat to reflux for 3h, pour into ice water, A white solid was precipitated, filtered and recrystallized to obtain 5.4 g of white crystals with a yield of 95%.
[0071] 1 H NMR (500MHz, CDCl 3 ),δ H , ppm: 0.73(s, 3H, 20-H 3 ), 0.96(s, 3H, 17-H 3 ), 1.16(s, 3H, 19-H 3 ), 1.20-1.22 (dd, 6H, CH (C H 3 ) 2 , J=6.27, 3.13Hz), 0.86-2.65 (m, 18H, 1-H 2 ,2-H 2 , 3-H ax , 5-H, 6-H 2 ,7-H 2 , 9-H, 11-H 2 , 12-H 2 , 14-H 2 , 15-H β ), 2.15-2.18 (d, 1H, 3-H eq , J=15.00Hz), 2.60-2.65 (dd, 1H, 15-H α , J=18.59, 3.86Hz), 4.93-4.98 (m, 1H, C H (CH 3 ) 2 )
Embodiment 3
[0072] Example 3: Bis((4α, 8β, 13β)-13-methyl-16-oxo-17-norkaurane-18-oic acid) methylene ester (I 3 )
[0073] Dissolve 0.64g (0.002mol) of isosteviol and 0.68g (0.002mol) of tetrabutylammonium hydrogensulfate in 5mL of 2M sodium hydroxide solution, extract the organic layer with dichloromethane, dry over anhydrous magnesium sulfate, filter, and keep the filtrate at room temperature After reacting for 24 hours, it was washed, dried over anhydrous magnesium sulfate, filtered, concentrated, and column chromatography gave 0.5 g of white solid with a yield of 77%.
[0074] 1 H NMR (300MHz, CDCl 3 ),δ H , ppm: 0.73 (s, 6H, 20, 20'-H 3 ), 0.98(s, 6H, 17, 17'-H 3 ), 1.20 (s, 6H, 19, 19'-H 3 ), 0.90-1.90 (m, 36H, 1, 1'-H 2 , 2,2′-H 2 , 3,3′-H ax , 5,5'-H, 6,6'-H 2 ,7,7′-H 2 ,9,9′-H,——11,11′-H 2 ,12,12′-H 2 , 14, 14′-H 2 ,15,15′-H β ), 2.15-2.19 (d, 2H, 3, 3'-H eq , J=13.47Hz), 2.58-2.66 (dd, 2H, 15, 15'-H α , J=18.59, 3.68Hz), 5.71(s, 2H, OC H 2 O)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com