Synthetic method for preparing rutile type nano titanic oxide sol or powder at low temperature
A nano-titanium dioxide, rutile technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
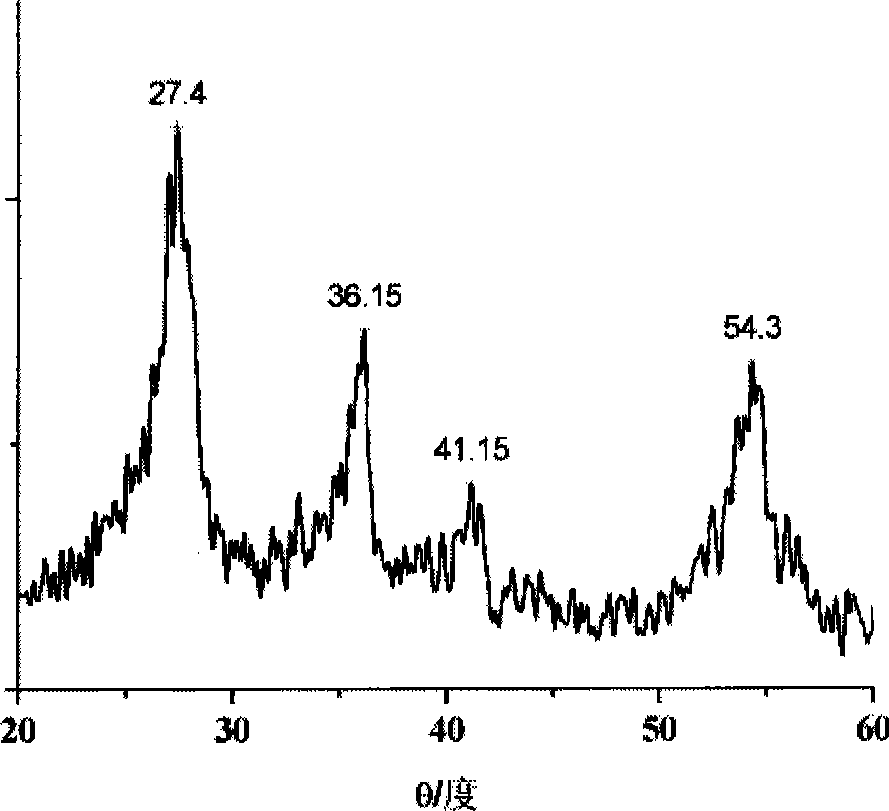
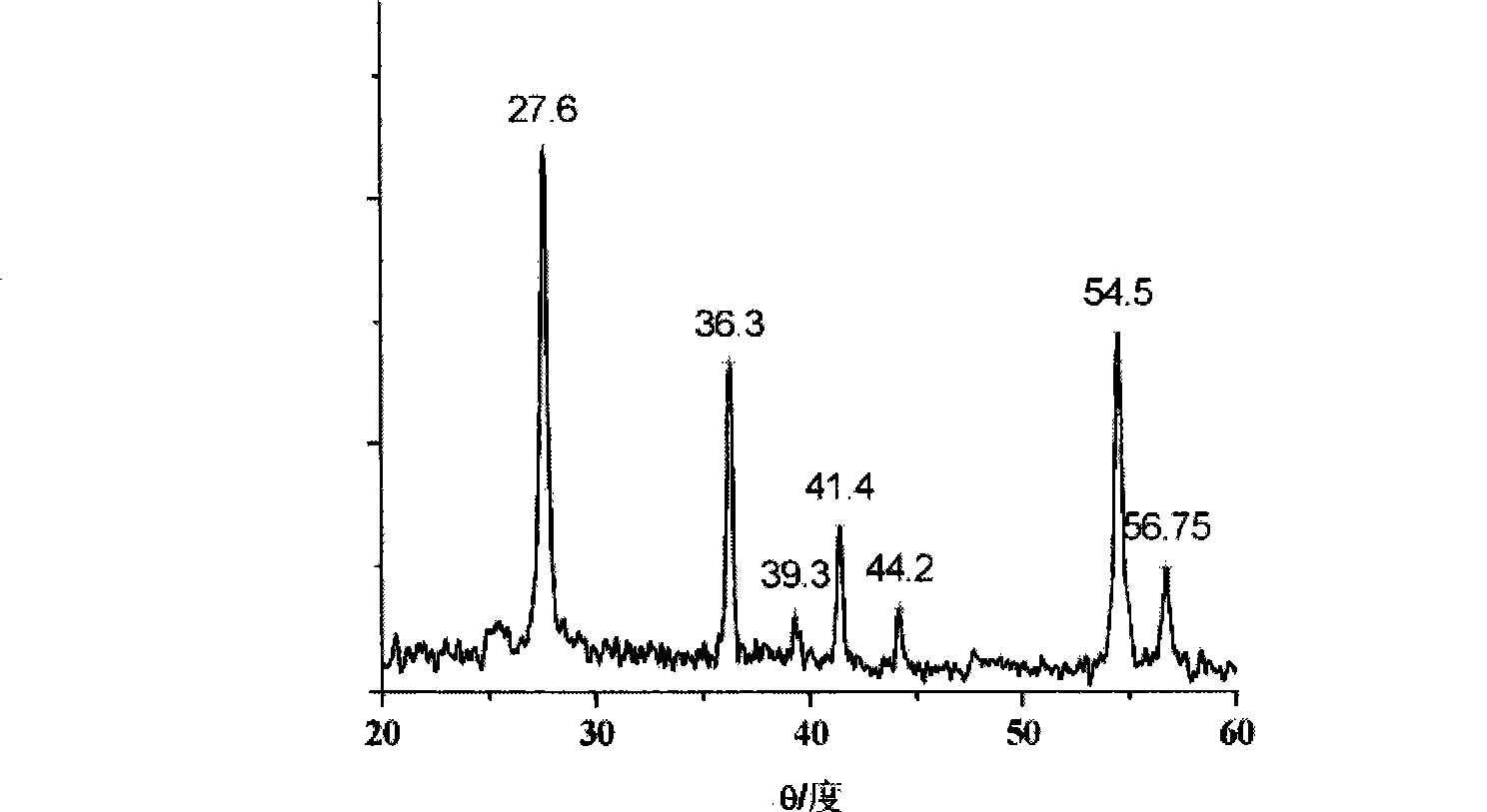
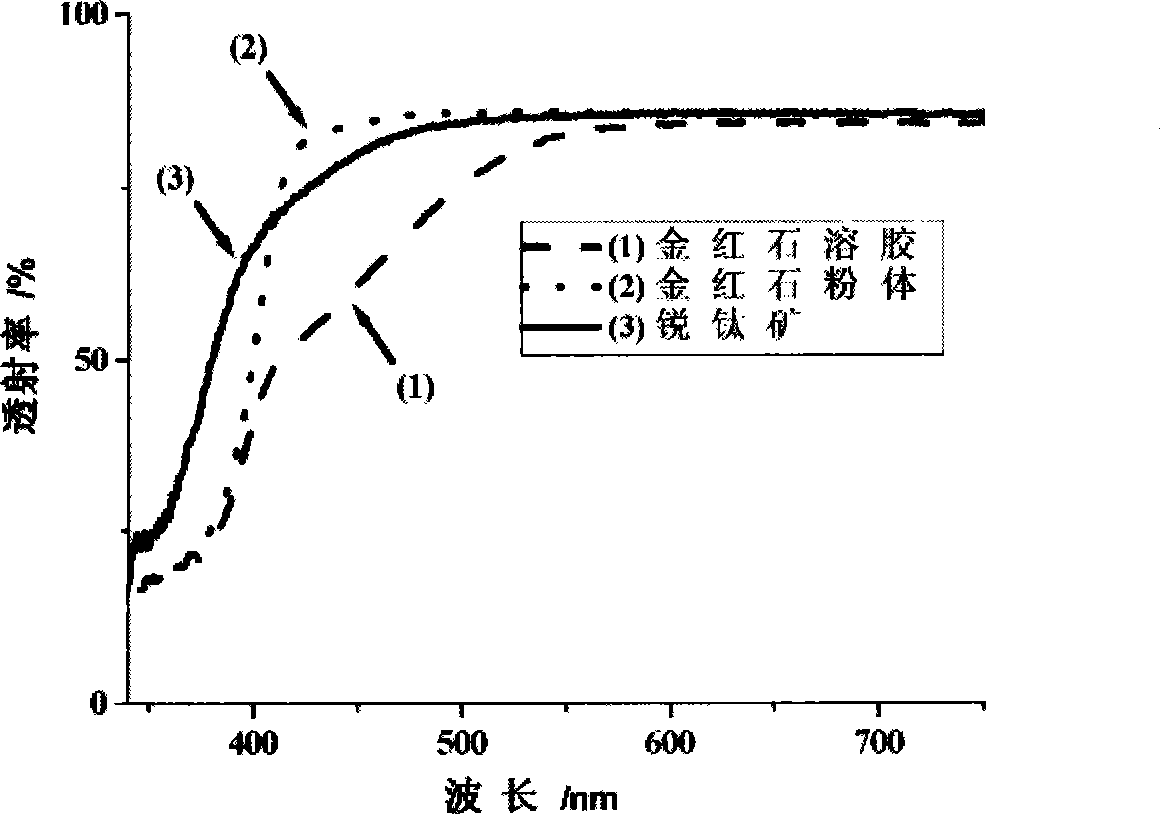
[0036] 1.5g SnCl 2 2H 2 O dissolved in 400mL 0.2mol / L TiCl 4 In the solution, the pH of the solution was adjusted to 8 with ammonia water, and a yellow composite orthotitanic acid precipitate was obtained. Disperse the precipitate in 80 mL of hydrogen peroxide solution with a mass concentration of 30%, add water to adjust the volume to 150 mL, adjust the pH value to 3 with nitric acid, and reflux at 100 ° C for 12 hours to obtain orange-yellow rutile nano-titanium dioxide sol, see Figure 4 . XRD characterizes as rutile nano titanium dioxide crystals, see figure 1 .
Embodiment 2
[0038] 0.9g SnCl 4 Dissolve in 400mL 0.2mol / L TiOSO 4 In the solution, dilute the solution with water to 50 times of the original volume to obtain composite orthotitanic acid precipitation. Disperse the precipitate in 120 mL of 30% hydrogen peroxide solution, add water to adjust the volume to 200 mL, adjust the pH to 6 with ammonia solution, and reflux at 50°C for 24 hours to obtain a yellow nano-titanium dioxide sol.
Embodiment 3
[0040] Sulfuric acid with a mass concentration of 80% is heated to dissolve ilmenite, and the obtained solid phase deposit is leached with water to obtain titanium liquid, the titanium liquid is filtered to remove insoluble slag, and frozen and recrystallized to filter and remove ferrous sulfate to obtain a titanium-containing precursor solution; 1.5gSnCl 2 2H 2 O was dissolved in 200 mL of precursor solution containing 0.4 mol / L titanium, heated and stirred at 100 °C to obtain composite orthotitanic acid precipitation. Disperse the precipitate in 40 mL of 30% hydrogen peroxide solution, add water to adjust the volume to 100 mL, adjust the pH to 2 with sulfuric acid, and reflux at 100° C. for 2 hours to obtain an orange-yellow nano-titanium dioxide sol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com