Polyaluminum calcium hydroxychlorides and methods of making the same
A technology of aluminum chloride and aluminum source, applied in the direction of aluminum chloride, chemical instruments and methods, preparation of alkaline earth metal aluminate/aluminum oxide/aluminum hydroxide, etc., capable of solving the problem of undisclosed polymerized calcium-aluminum hydroxychloride composition And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Example 1 : Preparation of PAC-Ca solution by adding aluminum chloride to the reaction product - Method A
[0033] Mix 191 parts of aluminum chloride with 269 parts of water heated to 80°C to 90°C, add 20 parts of calcium oxide (99% purity) and react to form a transparent solution, followed by adding aluminum powder. The reaction was terminated after 1.5 hours. After filtration a clear colorless solution was obtained with 6.03% Al, 3.0% Ca and 8.9% Cl. PAC-Ca solutions with different Al / Cl ratios and basicities were prepared by adding different amounts of AlCl solution to the above transparent solution. The results are listed in Table I.
Table I
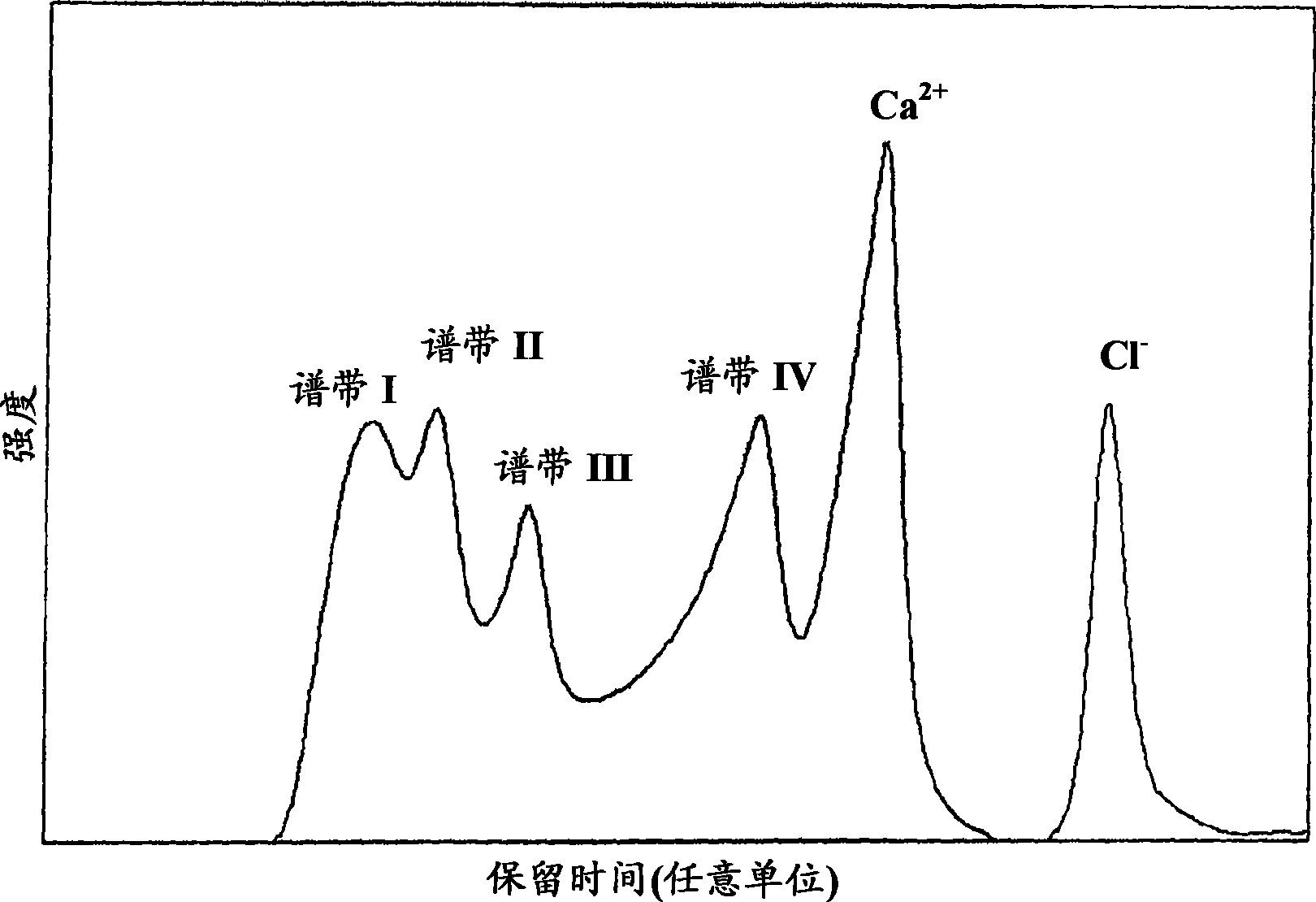
Experiment number Al / Cl ratio %Al %Ca % Alkalinity % Band I % Band IV 1 0.5 5.78 1.56 45.5 5.0 81.7 2 0.6 5.87 2.10 60.5 13.2 66.0 3 0.7 5.94 2.48 71.0 23.4 48.4 4 0.8 5.98 2.73 77.7 30.4 31.1
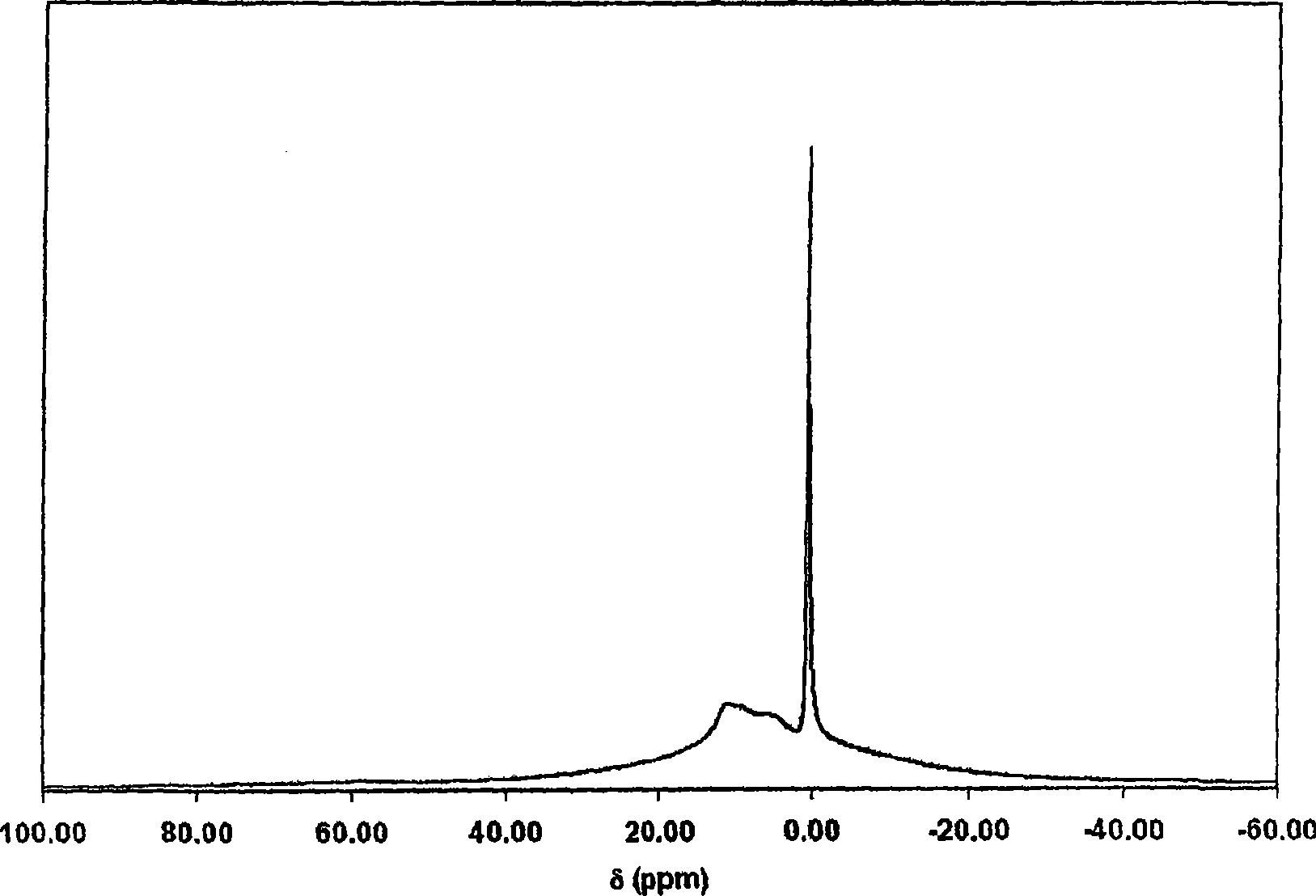
Note: It has been found that the larger polyaluminum species, represent...
Embodiment 2
Example 2 : Preparation of PAC-Ca solution by adding aluminum chloride to the reaction product - Method B
[0034] The different aluminum chloride solutions were added to the solution of the reaction product prepared as follows by mixing bauxite, calcium aluminate and aqueous hydrochloric acid at about 100°C and heating for the required period of time, cooling , settled, filtered and dried. The results of the obtained PAC-Ca solutions are listed in Table II.
Table II
Experiment number Al / Cl ratio %Al %Ca % Alkalinity % Band I % Band IV 5 0.50 7.0 2.66 50.5 5.4 86.5 6 0.60 7.0 2.73 61.9 16.3 60.8 7 0.70 7.0 3.25 72.9 27.0 32.6 8 0.76 7.0 346 78.0 33.6 313
Embodiment 3
Example 3: Comparison of PAC-Ca solutions prepared by adding acidic aluminum solution to the reaction product according to Method A at high and low temperatures
(i) Preparation of acidic aluminum solution
[0035] 785 parts of the aluminum chloride solution are mixed with 183 parts of water and heated. Add 32 parts of aluminum powder and react at 90°C to 95°C for about 1 hour. The reaction mixture was filtered to obtain a clear solution containing 7.6% Al and 17.5% Cl with an Al / Cl ratio of 0.57.
(ii) Preparation of PAC-Ca solution at low temperature
[0036] 106 parts of aluminum chloride, 123 parts of water and 8.75 parts of calcium oxide were mixed and refluxed for 1 hour to obtain an almost transparent solution, then the solution was cooled to about 55°C, and aluminum powder was added at this temperature within about 40 minutes. °C to 70 °C for 6.5 hours. The reaction mixture was filtered. 100 parts of this solution were mixed with 17 parts of the acidic aluminum s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com