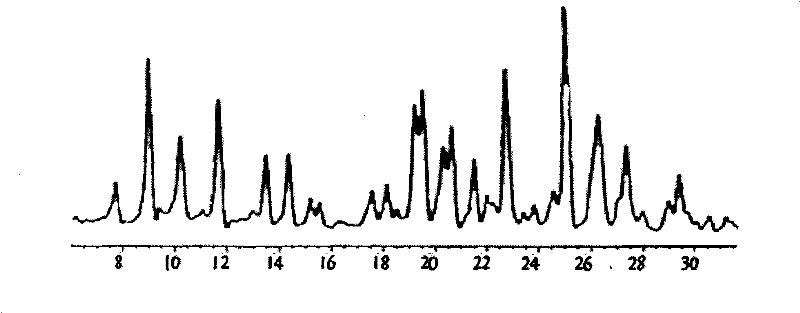
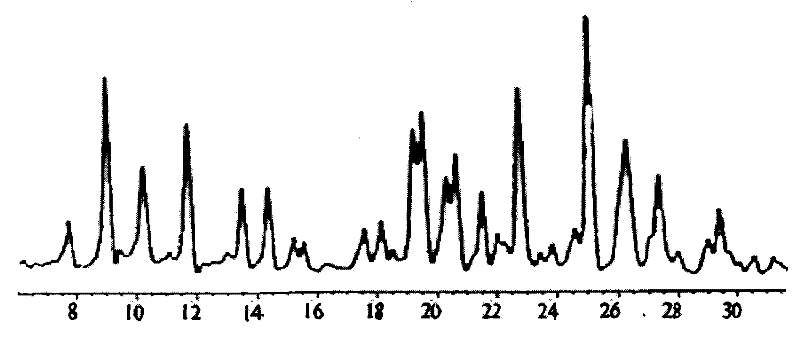
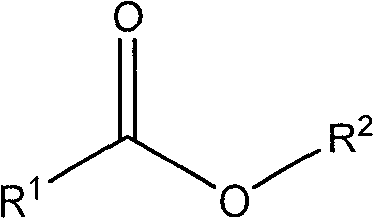
Method for preparing terbutaline sulphate crystal form B meeting medicinal requirements
一种硫酸特布他林、晶型的技术,应用在医药领域,能够解决未见明确披露制备硫酸特布他林晶型B方法等问题,达到化学及物理性质稳定的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 0.5 g of non-B crystalline terbutaline sulfate was stirred in 10 ml of aqueous ethyl acetate (saturated with water) at 25° C. for 24 hours, and then the resulting suspension was dried at 50° C. under nitrogen flow to constant weight. Terbutaline sulfate crystal form B was obtained.
Embodiment 2
[0034]0.5 g of non-B crystalline terbutaline sulfate was stirred in 10 ml of aqueous ethyl acetate (water half-saturated) at 25°C for 24 hours, and then the resulting suspension was dried at 50°C under nitrogen flow to constant weight. Terbutaline sulfate crystal form B was obtained.
Embodiment 3
[0036] 0.5 g of non-B crystalline terbutaline sulfate was stirred in 10 ml of aqueous ethyl acetate (saturated with water) at 35° C. for 24 hours, and then the resulting suspension was dried at 50° C. under nitrogen flow to constant weight. Terbutaline sulfate crystal form B was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com