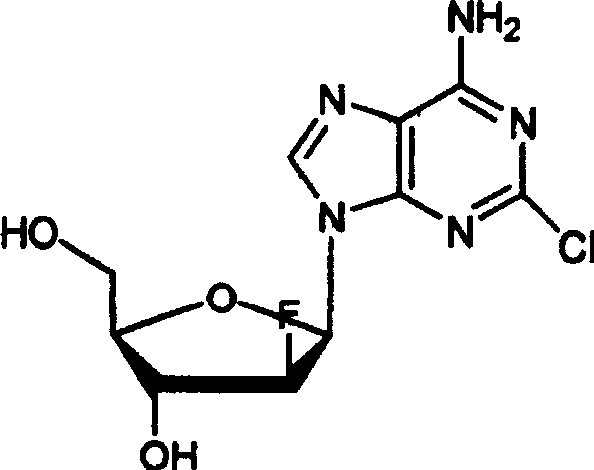
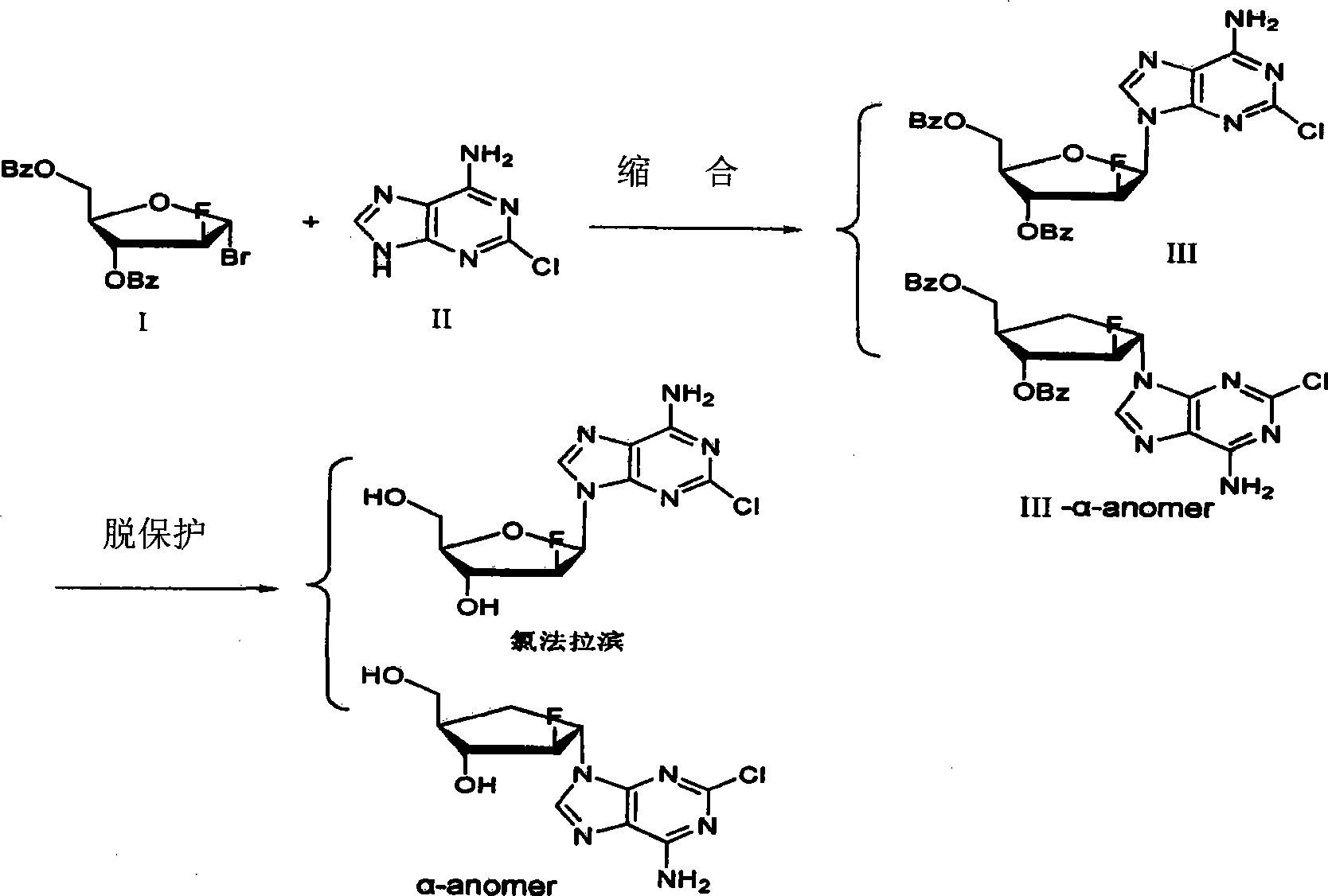
Method for purifying clofarabine by using chromatographic column
A clofarabine and chromatographic column technology, applied in the field of clofarabine purification, can solve problems such as being difficult to remove, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 600ml of MCI-gel CHP20P filler to a 2L beaker and soak in 1L of absolute ethanol for 10h. After stirring evenly, put it into a glass chromatographic column by wet method, the diameter of the chromatographic column is 5cm, and the length of the column is 100cm. Elute with 10L of purified water until the effluent has no ethanol, and the flow rate is controlled at 6ml / min. The time required is about 4h. Add 10 g of crude clofarabine to be purified into 4 L of purified water, and heat until completely dissolved. Cool naturally to room temperature and load the sample. The time required is about 2h. Use 5L purified water, 5L purified water and 250ml ethanol mixture, 5L purified water and 500ml ethanol mixture, 5L purified water and 750mL ethanol mixture, 5L purified water and 1L ethanol mixture to elute sequentially, and the flow rate is controlled at 6ml / min . The effluent was collected and tested for purity by HPLC. Collect clofarabine purity greater than 99.8%, t...
Embodiment 2
[0024] Add 600ml of MCI-gel CHP20P reverse packing to a 2L beaker, soak in 1L of absolute ethanol for 15h. After stirring evenly, put it into a glass chromatographic column by wet method, the diameter of the chromatographic column is 5cm, and the column length is 50cm. Elute with 10L of purified water until the effluent has no ethanol, and the flow rate is controlled at 6ml / min. Add 5 g of crude clofarabine to be purified into 4 L of purified water, and heat until completely dissolved. Cool naturally to room temperature and load the sample. The time required is about 2h. Use 5L purified water, 5L purified water mixed with 250ml methanol, 5L purified water mixed with 500ml methanol, 5L purified water mixed with 750mM methanol, 5L purified water mixed with 1L methanol, and the flow rate is controlled at 2ml / min . The effluent was collected and tested for purity by HPLC. The fractions of clofarabine with a purity greater than 99.8% and a single impurity less than 0.1% were c...
Embodiment 3
[0026] Add 600ml of MCI-gel CHP20SS reverse packing to a 2L beaker and soak in 1L of absolute ethanol for 15h. After stirring evenly, put it into a glass chromatographic column by wet method, the diameter of the chromatographic column is 5cm, and the column length is 50cm. Elute with 10L of purified water until the effluent has no ethanol, and the flow rate is controlled at 6ml / min. Add 2.5 g of crude clofarabine to be purified into 4 L of purified water, and heat until completely dissolved. Cool naturally to room temperature and load the sample. The time required is about 2h. Use 5L of purified water, 5L of purified water and 250ml of acetone mixture, 5L of purified water and 500ml of acetone mixture, 5L of purified water and 750mL of acetone mixture, 5L of purified water and 1L of acetone mixture, and the flow rate is controlled at 4ml / min . The effluent was collected and tested for purity by HPLC. The fractions of clofarabine with a purity greater than 99.8% and a sing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com