New method for salt preparation
A technology of hydrochloric acid and acid addition salt, which is applied to the preparation and crystallization of hydrohalides of pharmaceutical compounds or their intermediates, in the field of industrial application, which can solve the problems of preferential formation, uneasy control, difficult operation, etc., and achieve good yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Preparation of anhydrous crystal form of mycophenolate mofetil hydrochloride
[0245] 2 g (4.61 mmol) of mycophenolate mofetil base were dissolved in 50 ml of ethyl acetate at room temperature. To this solution were added 0.3 ml (1.2 equiv.) of acetic acid and 0.7 ml (1.2 equiv.) of trimethylchlorosilane with stirring. After 2 minutes at room temperature, crystallization started. The suspension was stirred for 1 hour and the precipitate was filtered off. The solid was washed with ethyl acetate and dried under vacuum at room temperature to afford 2.11 g (97.6%) of mycophenolate mofetil hydrochloride.
[0246] mp. = 157.2°C.
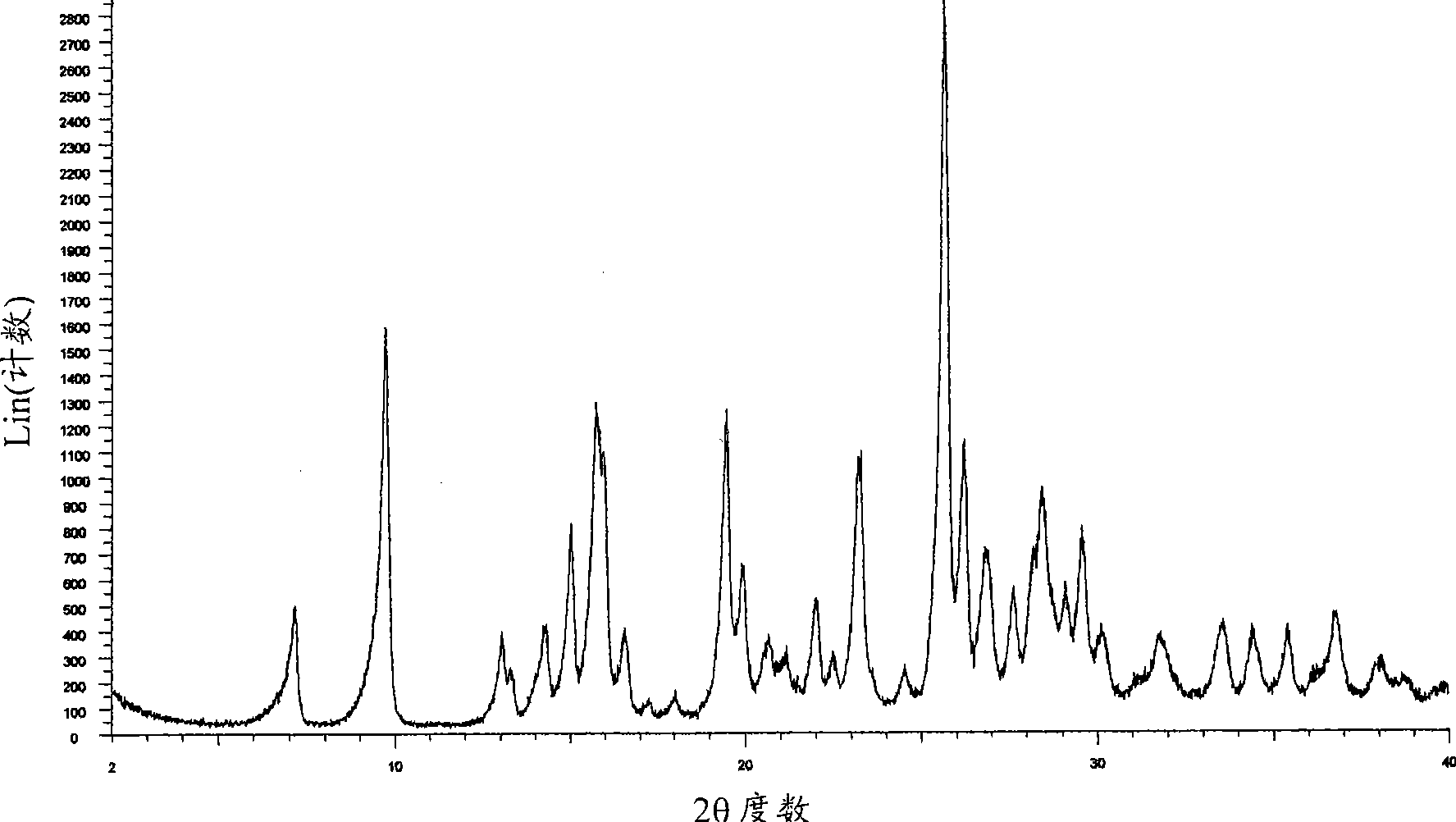
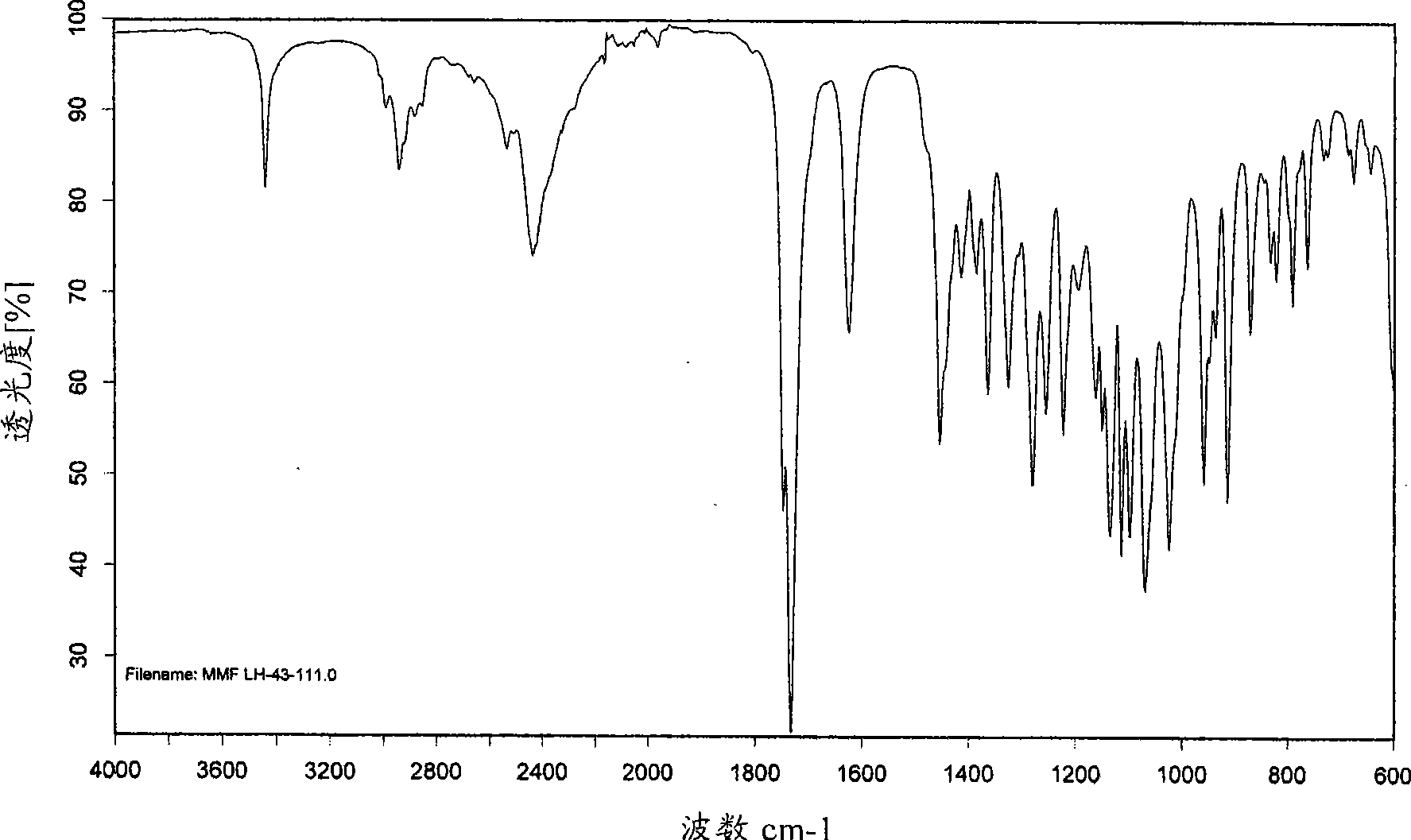
[0247] XRD pattern of mycophenolate mofetil hydrochloride Figure 1A and corresponds to the anhydrous crystalline form with X-ray crystallographic data as shown in WO95 / 07902. The infrared spectrum obtained as Figure 1B shown in .
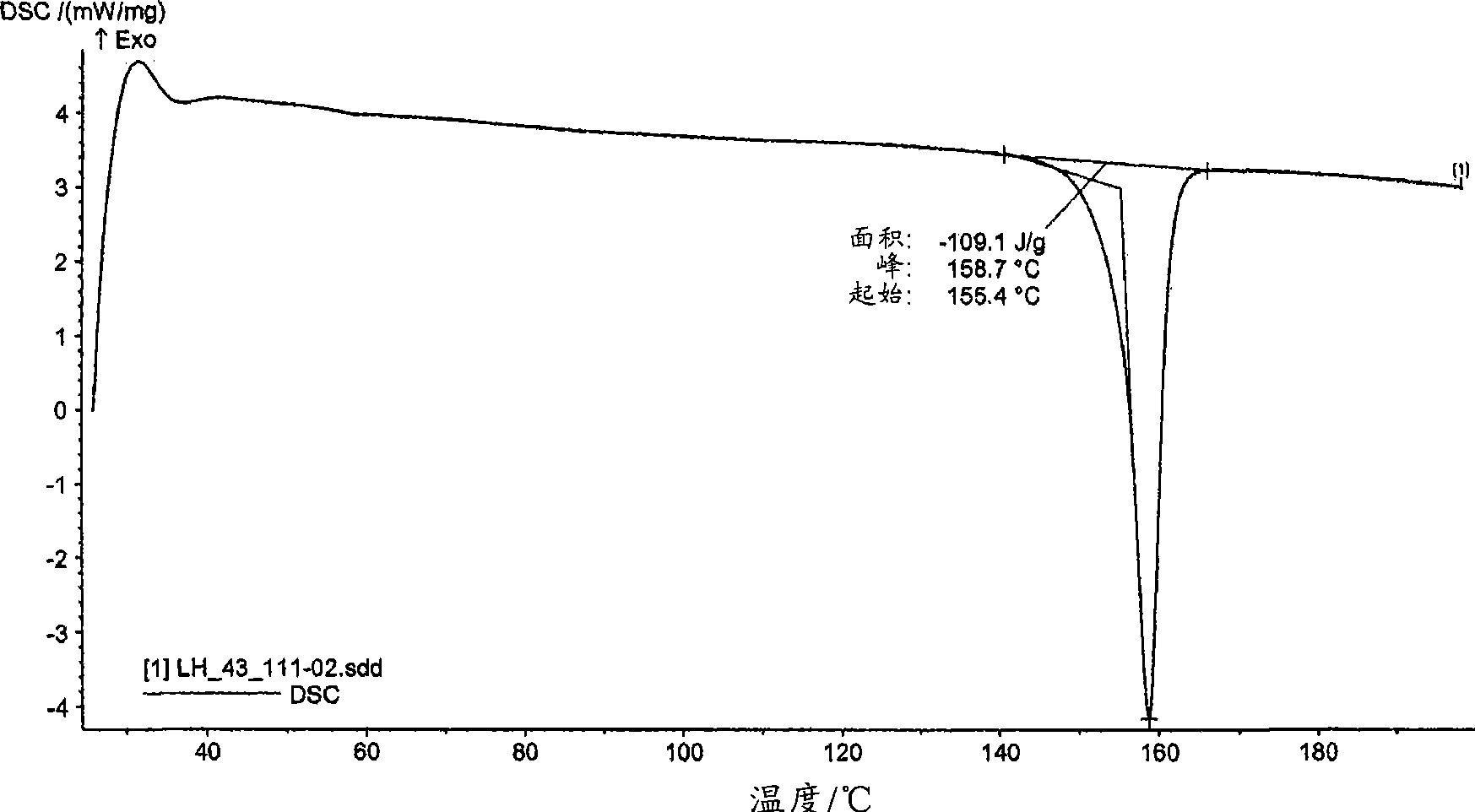
[0248] The DSC of mycophenolate mofetil hydrochloride shows an endothermic peak at about 159°C (onset temperatu...
Embodiment 2
[0250] Preparation of venlafaxine hydrochloride
[0251] The preparation of embodiment 2.a venlafaxine hydrochloride crystal form I
[0252] 0.4 g (1.44 mmol) of venlafaxine base were dissolved in 10 ml of ethyl acetate at room temperature. To this solution was added 0.1 ml (1.1 equiv.) of acetic acid and 0.2 ml (1.1 equiv.) of trimethylchlorosilane with stirring. After 2 minutes at room temperature, crystallization started. The suspension was stirred for 30 minutes and the precipitate was filtered off. The solid was washed with ethyl acetate and dried under vacuum at room temperature to afford 0.41 g (89.1%) of venlafaxine hydrochloride.
[0253] mp. = 208°C.
[0254] XRD pattern of venlafaxine hydrochloride crystal form I Figure 2A and corresponds to Form I with X-ray crystallographic data as shown in US03 / 0114536.
[0255] The infrared spectrum obtained as Figure 2B shown in .
[0256] Embodiment 2.b Preparation of venlafaxine hydrochloride crystal form II
[025...
Embodiment 3
[0264] Preparation of Sertraline Hydrochloride Form II Using Sertraline Base
[0265] Example 3.a
[0266] 3 g (9.8 mmol) sertraline base was dissolved in 60 ml acetonitrile at room temperature. To this solution were added 0.6 ml (1 eq) of acetic acid and 1.4 ml (1.1 eq) of trimethylchlorosilane with stirring. Sertraline hydrochloride precipitated out exactly in crystalline form II at the same time as the addition. After stirring the suspension for 1 hour, the product was filtered off and dried at 50° C. for 3 hours to afford 3.2 g (95.3%) of crystalline sertraline hydrochloride Form II.
[0267] mp.: 252°C.
[0268] The obtained XRD pattern is as follows Figure 4A shown in and corresponds to pure Form II.
[0269] The infrared spectrum obtained as Figure 4B shown in .
[0270] Example 3.b
[0271] 3 g (9.8 mmol) of sertraline base were dissolved in a mixture of 60 ml of acetonitrile and 1 ml of n-butanol. The solution was heated to 50 °C and 1.4 ml (1.1 eq) trimeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com