Method for synthesizing thymosins
A technology of thymosin and thymus, applied in the field of biomedical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
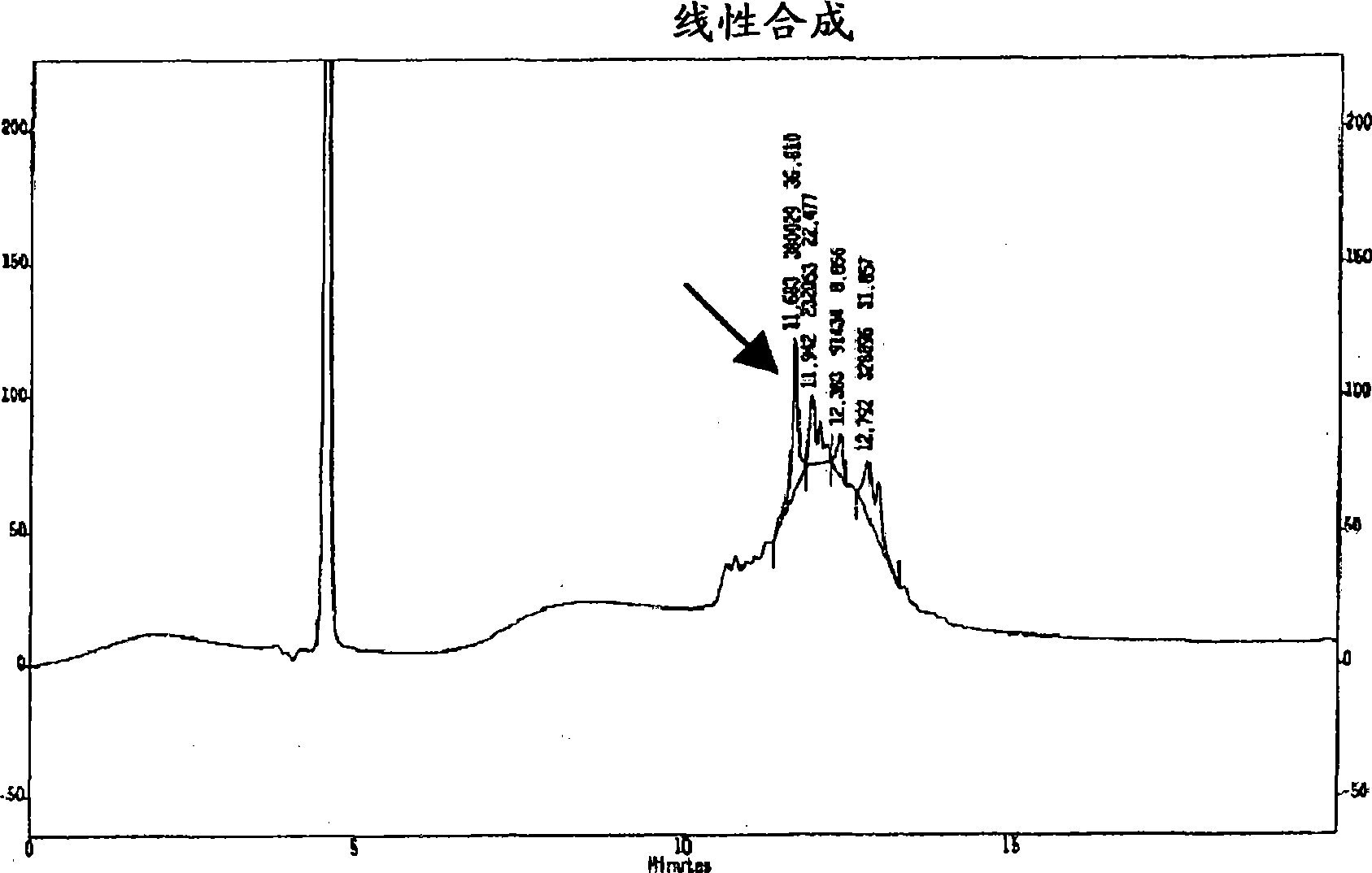
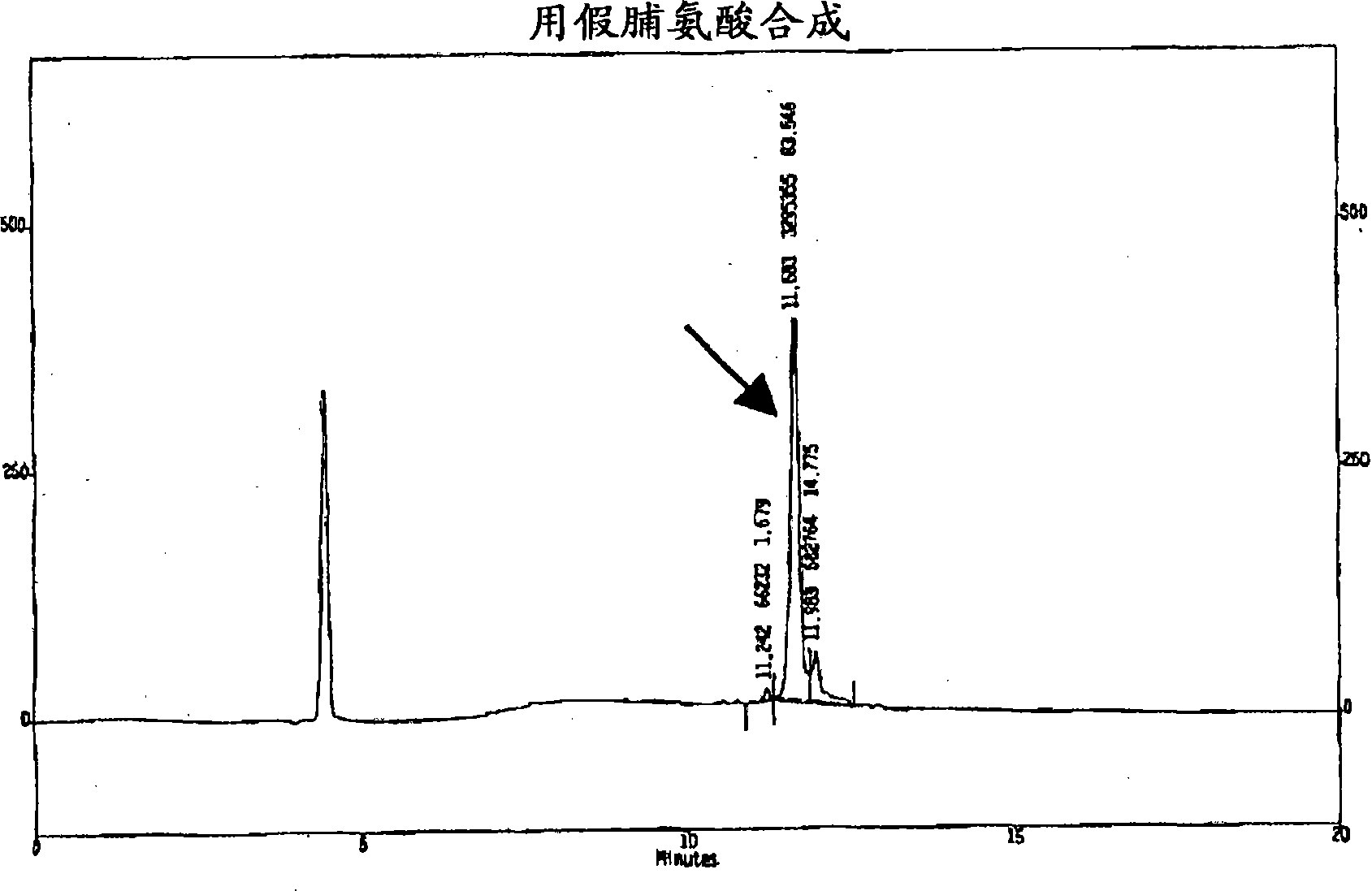
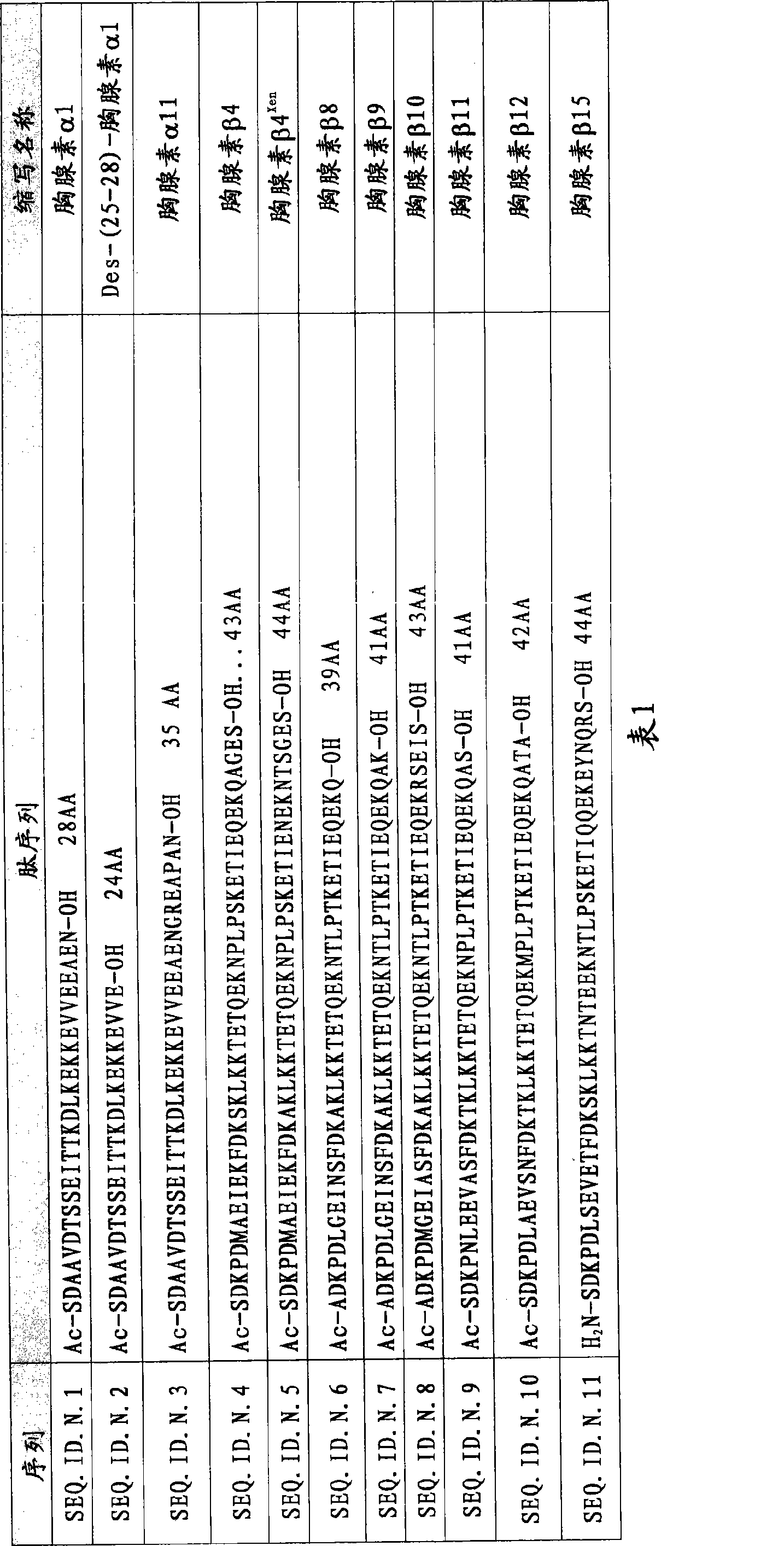
[0137] Example 1: Synthesis of Thymosin α1
[0138]The Fmoc-Asn-OH C-terminal residue was introduced on 2-chlorotrityl resin lacking amino acids to obtain 0.85 mmol / g functionalization after the introduction of the first amino acid. 0.8 g of amino acids (2.13 mmol, 0.5 eq.) were introduced on 2.5 g of resin (f = 1.6 mmol / g resin, 4 mmol). Resin and amino acid were weighed in separate containers and allowed to dry under vacuum for at least 4 hours. Dissolve the amino acid in 25 mL of dry DCM (at 4 sieve). DIEA (0.7 mL, 1.6 mmol, 1 eq.) was added and it was stirred for 5 minutes. A 1:1 solution (2.8 ml) of DIEA:DCM (1.4 mL, 3.2 mmol, 2 eq.) was prepared and added to the reaction mixture. It was stirred for another 40 minutes, then 4 mL of dry MeOH was added and allowed to react for 10 minutes, after which time the resin was filtered in a synthesis reactor equipped with a filter plate and a key, with the following washes:
[0139] Step Reagent Repeat Time
[0140] 1 DCM ...
Embodiment 2
[0154] Example 2: Synthesis of Thymosin β4
[0155] Thymosin β4 was synthesized using a solid-phase peptide synthesis strategy using Fmoc / tBu protection. For the introduction of the first amino acid on 2-chlorotrityl resin (1 g, 1.6 mmol / g), a DCM solution of Nα-Fmoc-protected amino acid (0.5 eq) and DIEA (3 eq.) was used to A functionalization of 0.85 mmol / g was achieved after amino acid. It was mechanically stirred for 40 min, then 4 mL of dry MeOH was added and allowed to react for 10 min. The resin was filtered in a synthesis reactor equipped with a filter plate and a key, and the following washes were performed with DCM and DMF. The resin was treated with 5% piperidine in DMF for 1 x 10 min and 20% piperidine in DMF for 1 x 15 min and rinsed with DMF. Once the first amino acid has been introduced, the remaining amino acids are Na-Fmoc-protected amino acids (3 eq.), HOBT (3 eq.) and DIPCDI (3 eq.) in DMF, the equivalents being calculated relative to the functionalizat...
Embodiment 3
[0163] Example 3: Synthesis of Thymosin β15
[0164] The synthesis method is similar to that used for thymosin β4. Using Fmoc-Lys(Boc)-Ser(Ψ Me,Me pro)-OH dipeptide replaces amino acids 14-15 with Fmoc-Asn(Trt)-Thr(Ψ Me,Me pro)-OH, and Fmoc-Glu(OtBu)-Thr(Ψ Me,Me pro)-OH. Coupling is only required at Lys(3).
[0165] The peptidyl resin Ser(tBu)-Asp(OtBu)-Lys(Boc)-Pro-Asp(OtBu)-Leu-Ser(tBu)-Glu(OtBu)-Val-Glu(OtBu)- Thr(tBu)-Phe-Asp(OtBu)-Lys(Boc)-Ser(Ψ M e,Mepro)-Lys(Boc)-Leu-Lys(Boc)-Lys(Boc)-Thr(tBu)-Asn(Trt)-Thr(Ψ Me,Me pro)-Glu(OtBu)-Glu(OtBu)-Lys(Boc)-Asn-Thr(tBu)-Leu-Pro-Ser(tBu)-Lys(Boc)-Glu(OtBu)-Thr(Ψ Me,Me pro)-Ile-Gln-Gln-Glu(OtBu)-Lys(Boc)-Glu(OtBu)-Tyr(tBu)-Asn-Gln-Arg(Pbf)-Ser(tBu)-resin.
[0166] By making it with TFA:H 2 The O 95:5 mixture was reacted for 1 hour 30 minutes to cleave the peptide from the resin, precipitate it with diethyl ether and lyophilize it. The crude product was purified by semi-preparative RP-HPLC and the final product was char...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com