Preparation of Azelnidipine alpha crystal form
A technique for the crystal form of azedipine, which is applied in the field of crystal form conversion of organic compounds, can solve the problems of literature or patents on the preparation method of α crystal form, and achieve the effects of good quality, easy recycling, and reduced manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of Azeldipine α Crystal Form
[0020] Take 20g of crude Azedipine, dissolve it in 20ml of ethyl acetate and 150ml of benzene (when dissolving, first mix the two organic solvents, and then add Azedipine), heat it in a water bath to 40°C, add 200ml of cyclohexane, 0.1 g seed crystals, cooled to 20° C. for crystallization, and the precipitated crystals were collected with a yield of 85%.
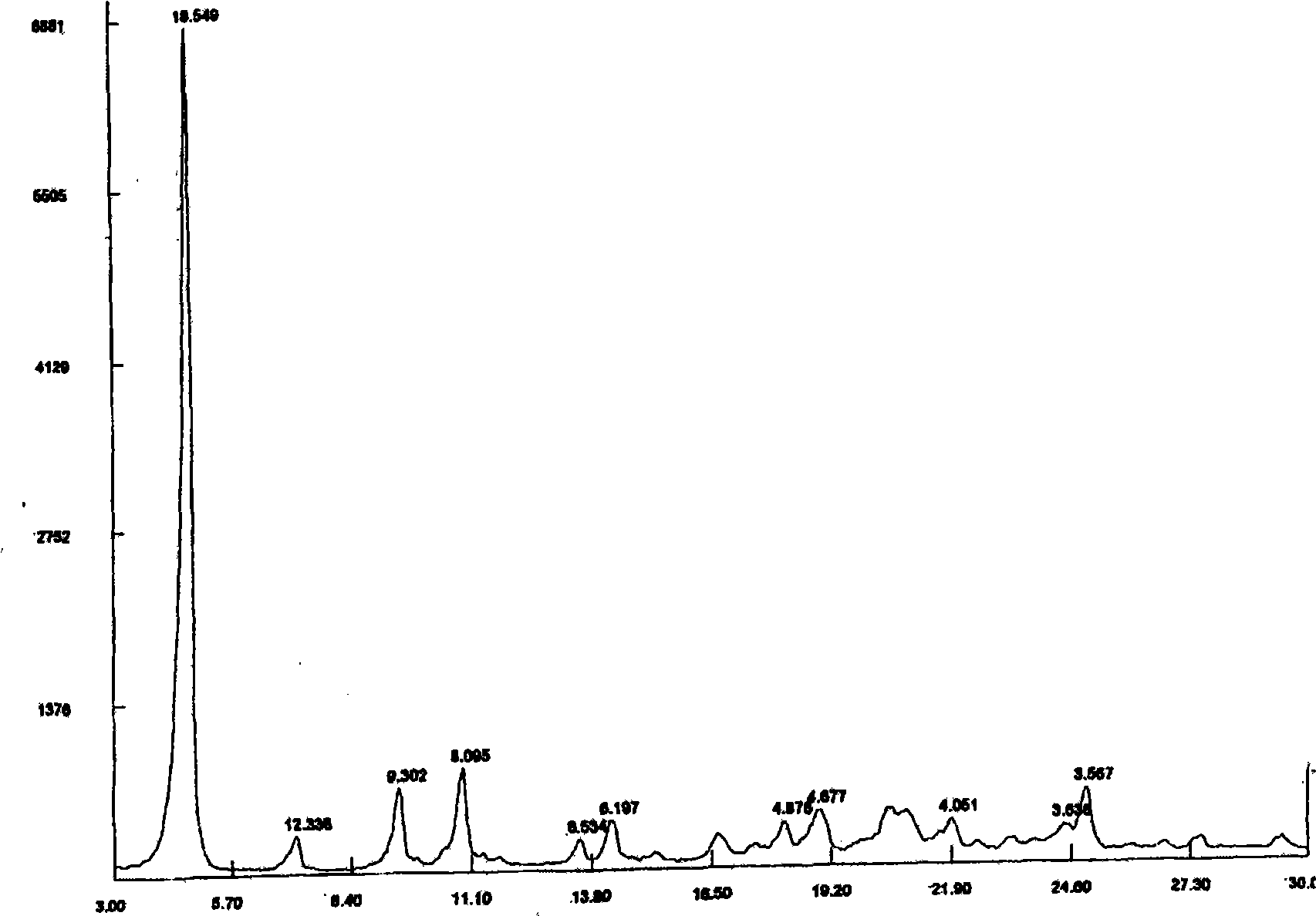
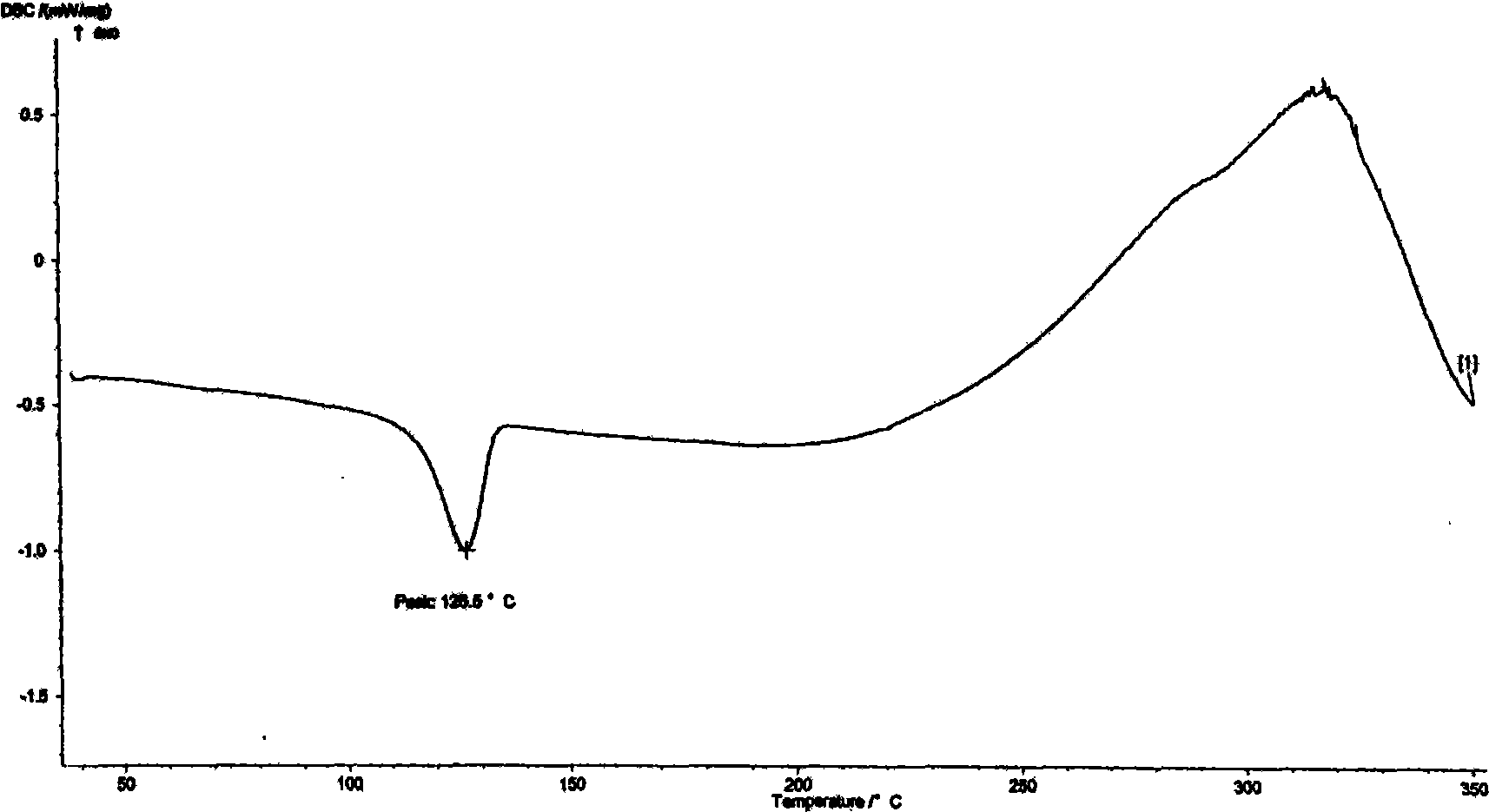
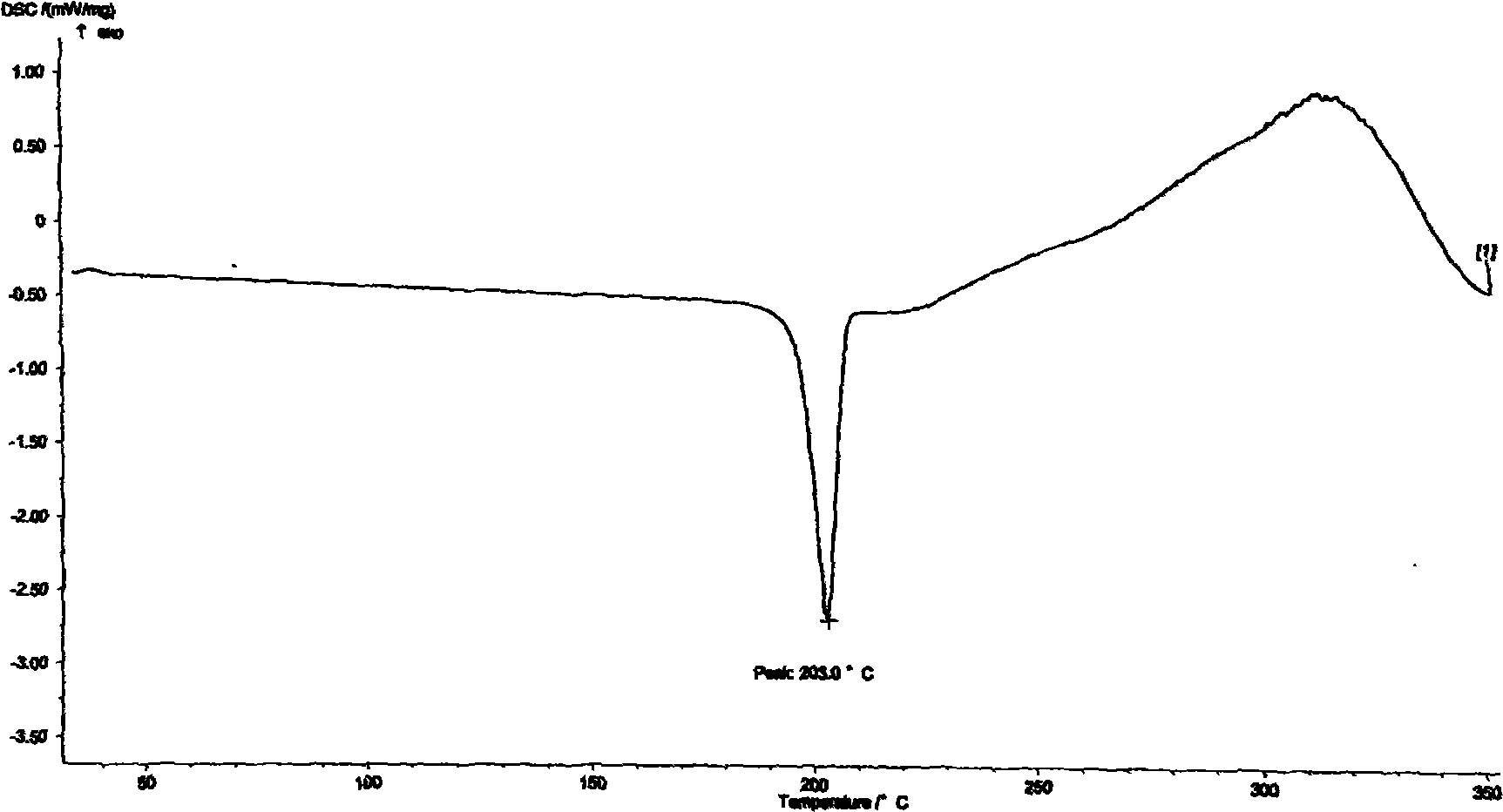
[0021] Carry out DSC analysis to the Azeldipine α crystal form prepared by the above-mentioned method and the crude product of Azeldipine, the results are as follows: figure 2 , image 3 shown. Carry out X-ray diffraction analysis to the Azeldipine α crystal form that above-mentioned method prepares, the result is as follows figure 1 shown. As can be seen from the figure, the α-crystal form of azeldipine prepared by the method of the present invention has high purity and good quality.
Embodiment 2
[0022] Example 2: Preparation of Azeldipine α Crystal Form
[0023] The water bath was heated to 45° C., and the rest was the same as in Example 1, and the final yield was 87%.
Embodiment 3
[0024] Example 3: Preparation of Azeldipine α Crystal Form
[0025] The water bath was heated to 50° C., and the rest was the same as in Example 1, and the final yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com