Bioactive purified hspe7 compositions
A technology of biological activity and composition, applied in the field of immunology, can solve problems such as poor immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Preparation of HspE7
[0101] The Hsp65-E7 fusion (HspE7) was obtained as described in WO99 / 07860 (which is incorporated herein by reference). HspE7 is a fusion protein that includes the entire HPV16E7-coding region inserted at the carboxy-terminal end of the Hsp65 gene (pET65H). This HspE7 is called method AHspE7, and can be obtained from Nventa Biopharmaceuticals Corporation as required.
[0102]Before use, HspE7 was purified to a purity higher than 95%. A seed culture of E. coli expressing HspE7 was used to inoculate 250 L of fermentation medium. During the fermentation process, yeast extract and glucose are added as nutrients, and pure oxygen is emitted into the fermentation vessel to provide sufficient ventilation. The expression of HspE7 was induced by adding IPTG (isopropyl-β-D-thiogalactopyranoside). The contents of the fermenter are then cooled to below 20 and the cell paste is collected by centrifugation. The cytoplasm is resuspended in a buffer contain...
Embodiment 2
[0104] Example 2: Determination of the biological activity of HspE7 preparations
[0105] Antigen-specific stimulation of IFN-γ production by splenocytes: ELISPOT analysis
[0106] In the presence of E7 peptide by ELISPOT (Asai, T. et al., 2000, Clin. Diagn. Lab. Immunol. 7(2): 145-154), HspE7 induction of E7-specific CD8-positive T lymphocytes ( The enhancement of the ability of IFN-γ to produce cells): The mice were immunized with HspE7 (with or without adjuvant) subcutaneously at the nape of the neck in a total volume of 200 μl. After five to seven days, the mice were sacrificed, and their spleens were taken and processed into a single cell suspension. The cells were seeded in complete RPMI on a microporous filter plate pre-coated with anti-mouse IFN-γ antibody. The microfiltration plate was incubated at 37 for 20 hours. The cells were washed away, and IFN-γ spots were detected by incubating the microporous filter plate with the biotinylated secondary antibody mouse IFN-γ antib...
Embodiment 3
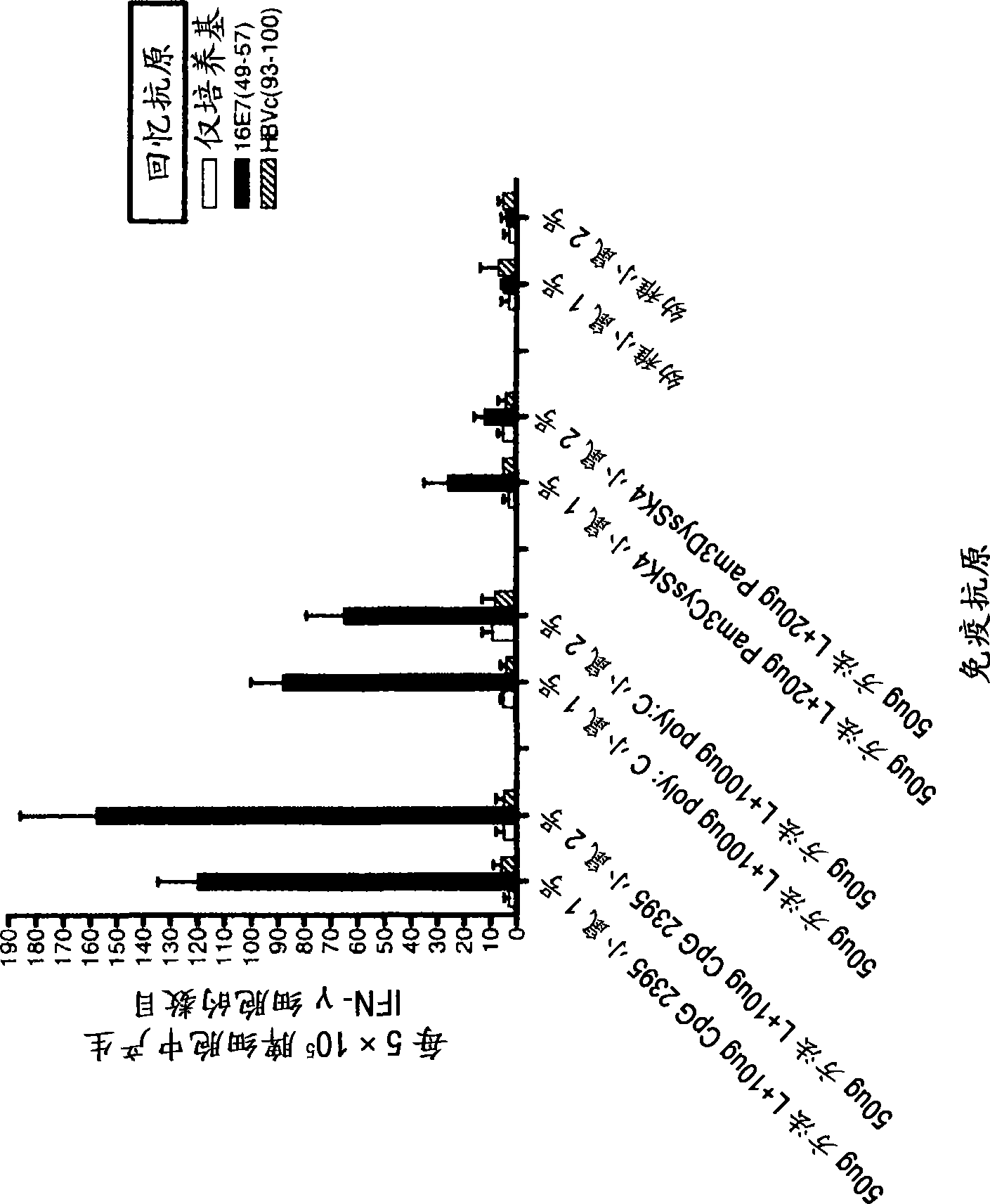
[0110] Example 3: The effect of TLR9 agonist CpG on HspE7
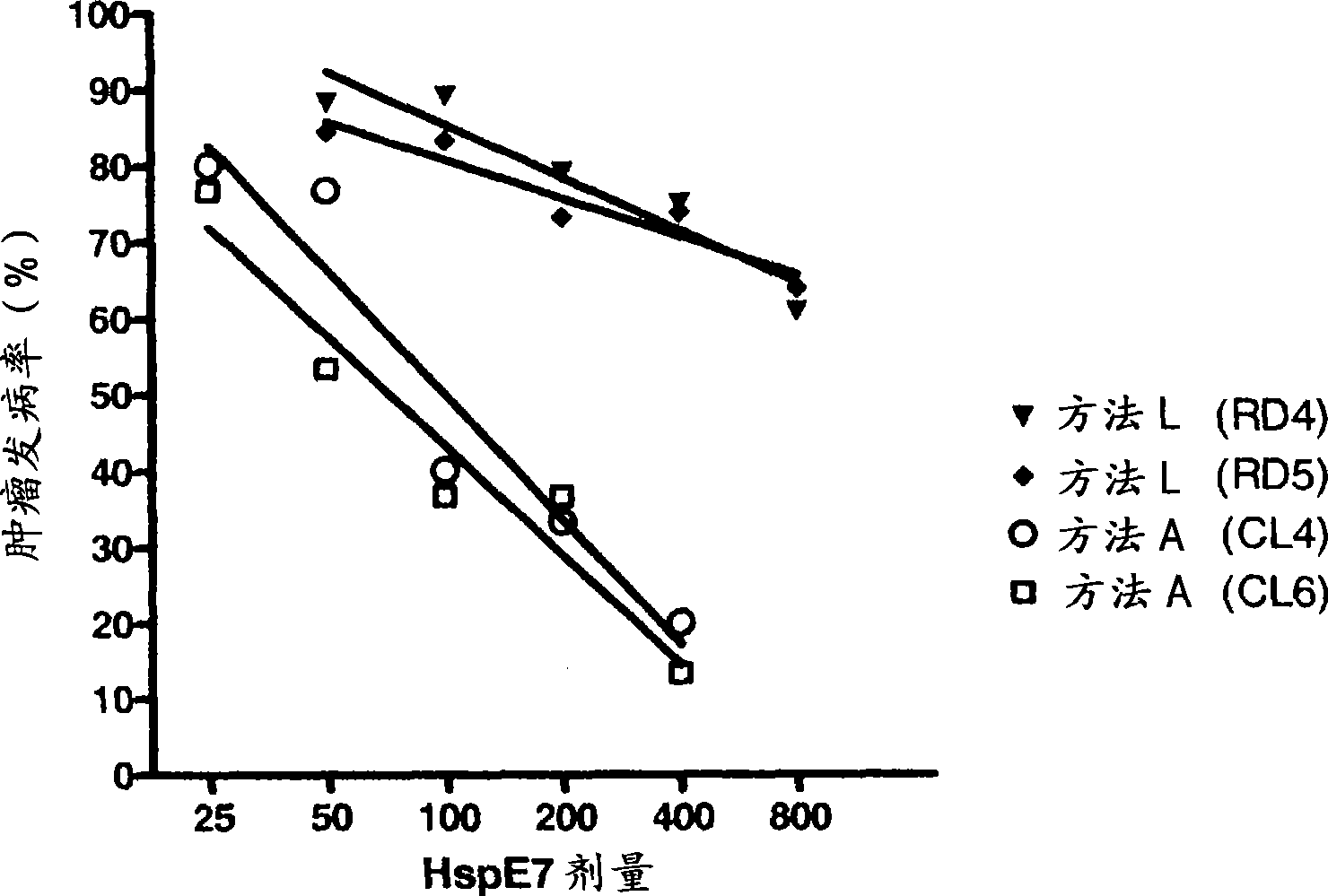
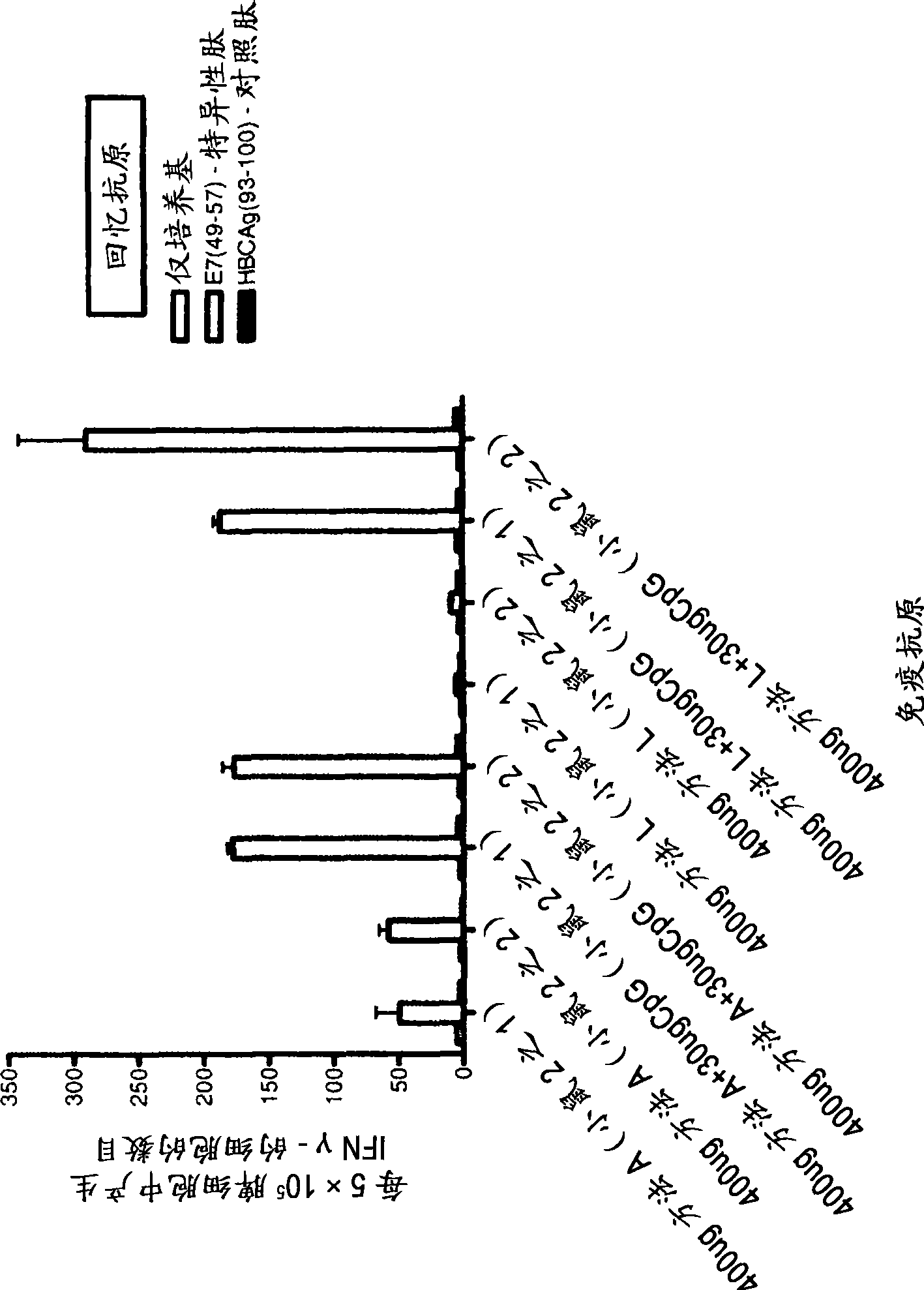
[0111] The enhancement of the ability of HspE7 to induce E7-specific CD8-positive T lymphocytes was determined in the presence of CpG oligonucleotides (TLR9 agonists). Use separate HspE7 (400μg method A HspE7 or 400μg method L HspE7) or HspE7 (400μg method A HspE7 or 400μg method L HspE7) prepared by two different purification methods plus 30μg of CpG (TCC ATG ACG TTC CTG ATG CT; SEQ ID NO: 1; obtained from Invitrogen, including phosphorothioate backbone and designated as: ZOO FZE FOE ZZO OZE FZE OT) Naive C57B1 / 6 mice were injected subcutaneously as described in Example 2. Five days later, the spleen was removed from the mouse and used E7 specific class I MHC binding peptide E7 49-57 (RAHYNIVTF; Dalton Chemical Laboratories) or control peptide HBCAg 93-100 (MGLKFRQL; Dalton Chemical Laboratories) was used as a recall antigen to measure the number of E7-specific splenocytes by ELISPOT.
[0112] figure 2 The results sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com