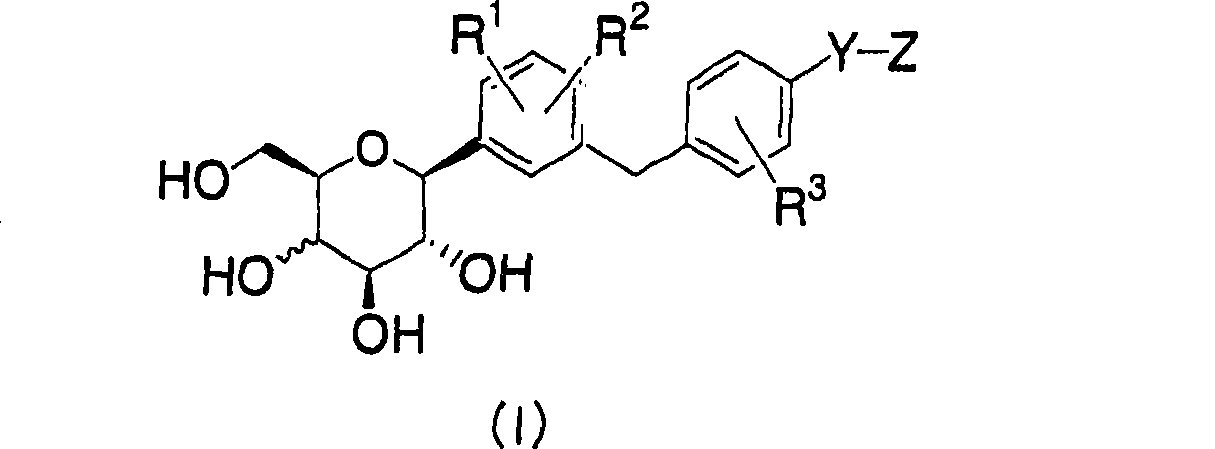
C-phenyl glycitol compound for the treatment of diabetes
A kind of technology of phenyl polyhydric alcohol and compound, applied in the field of C-phenyl polyhydric alcohol compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 2-19
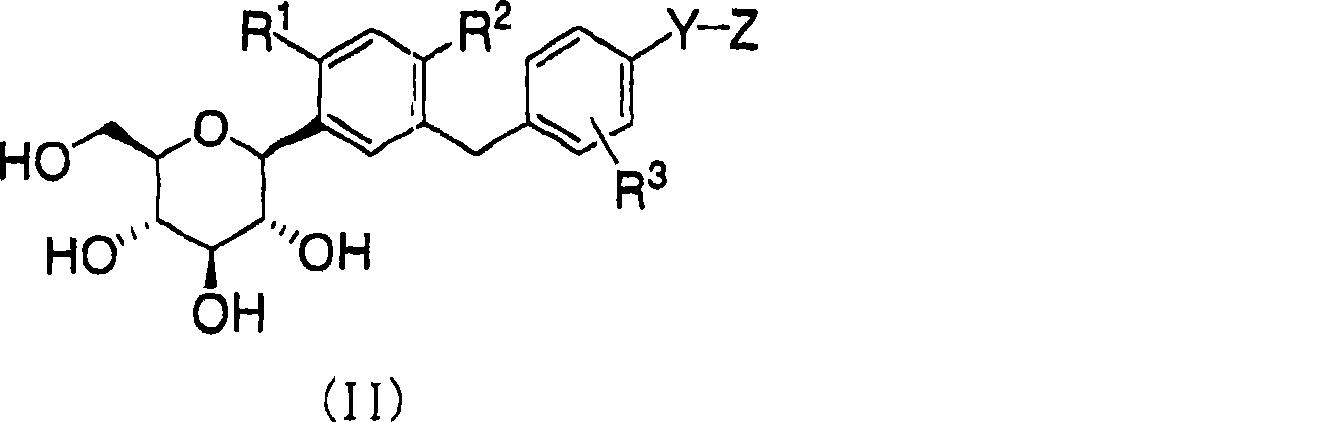
[0045] 2. The C-phenylpolyhydroxylitol compound, which is a C-phenylglucitol compound represented by the following formula (II) or a pharmaceutically acceptable salt thereof, or a hydrate thereof,
[0046]
[0047] where R 1 ,R 2 ,R 3 , Y and Z are the same as those defined in formula (I).
[0048] 3. The C-phenylpolyhydroxylitol compound of formula (II) or a pharmaceutically acceptable salt or hydrate thereof, wherein R 1 is a hydrogen atom, hydroxyl, C 1-4 Alkyl or C 1-4 Alkoxy, R 2 is C 1-4 alkyl or halogen atom.
[0049] 4. The C-phenyl polyhydroxylitol compound or its pharmaceutically acceptable salt or its hydrate according to embodiment 2 or 3, wherein R 3 is a hydrogen atom.
[0050] 5. The C-phenyl polyhydroxylitol compound or its pharmaceutically acceptable salt or its hydrate according to embodiment 3 or 4, wherein Y is C 1-6 Alkylene or -O-(CH 2 )n-(n is an integer of 2-4), Z is -NHCON(R B )R C , where R B and R C as defined in formula (I).
[00...
Embodiment 1
[0512] (1S)-1,5-anhydro-1-[2-hydroxy-5-[4-[4-[(2-hydroxy-1,1-dimethylethyl)amino]-4-oxobutyl Preparation of ]benzyl]-4-methylphenyl]-D-sorbitol
[0513]
[0514] (1) (1S)-1,5-anhydro-2,3,4,6-tetra-O-benzyl-1-[2-(benzyloxy)-5-[4-[(1E)-4 -[(2-Hydroxy-1,1-dimethylethyl)amino]-4-oxobut-1-en-1-yl]benzyl]-4-methylphenyl]-D-sorbose Preparation of Alcohol
[0515] To (1S)-1,5-anhydro-2,3,4,6-tetra-O-benzyl-1-[2-(benzyloxy)-5-[4-[(1E)-3-carboxy Prop-1-en-1-yl]benzyl]-4-methylphenyl]-D-sorbitol (200 mg, 0.223 mmol) in chloroform (2.2 mL) was added to 2-amino-2-methyl- 1-propanol (40 mg, 0.446 mmol), 1-hydroxybenzotriazole (33 mg, 0.245 mmol) and WSC (60 mg, 0.312 mmol), and the mixture was stirred at room temperature overnight. Water was added to the reaction solution, and the resulting mixture was extracted with chloroform. The organic layer was washed with brine, dried over anhydrous magnesium sulfate, the desiccant was removed by filtration, and the solvent was evaporated und...
Embodiment 2
[0521] (1S)-1,5-anhydro-1-[2-hydroxy-5-[4-[4-[[2-hydroxy-1-(hydroxymethyl)-1-methylethyl]amino]-4 Preparation of -oxobutyl]benzyl]-4-methylphenyl]-D-sorbitol
[0522]
[0523] (1) (1S)-1,5-anhydro-2,3,4,6-tetra-O-benzyl-1-[2-(benzyloxy)-5-[4-[(1E)-4 -[[2-Hydroxy-1-(hydroxymethyl)-1-methylethyl]amino]-4-oxobut-1-en-1-yl]benzyl]-4-methylphenyl] -Preparation of D-sorbitol-
[0524] According to the method described in Example 1 (1), but wherein 2-amino-2-methyl-1,3-propanediol is used instead of 2-amino-2-methyl-1-propanol, the title compound (91 mg, 44%), which is a colorless oily compound.
[0525] 1H NMR (300MHz, chloroform-D) δ ppm 1.19(s, 3H) 2.20(s, 3H) 3.15(d, J=6.06Hz, 2H) 3.49-3.83(m, 10H) 3.87-4.04(m, 3H) 4.37-4.67 (m, 4H) 4.80-4.94 (m, 3H) 5.00 (s, 2H) 6.00-6.23 (m, 2H) 6.40-6.52 (m, 1H) 6.75 (s, 1H) 6.93 (dd, J = 7.38, 1.94Hz, 2H) 7.03 (d, J = 8.24Hz, 2H) 7.11-7.35 (m, 24H) 7.35-7.46 (m, 2H).
[0526] ESI m / z=1004.5(M+Na).
[0527] (2) (1S)-1,5-anhydro-1-[2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com