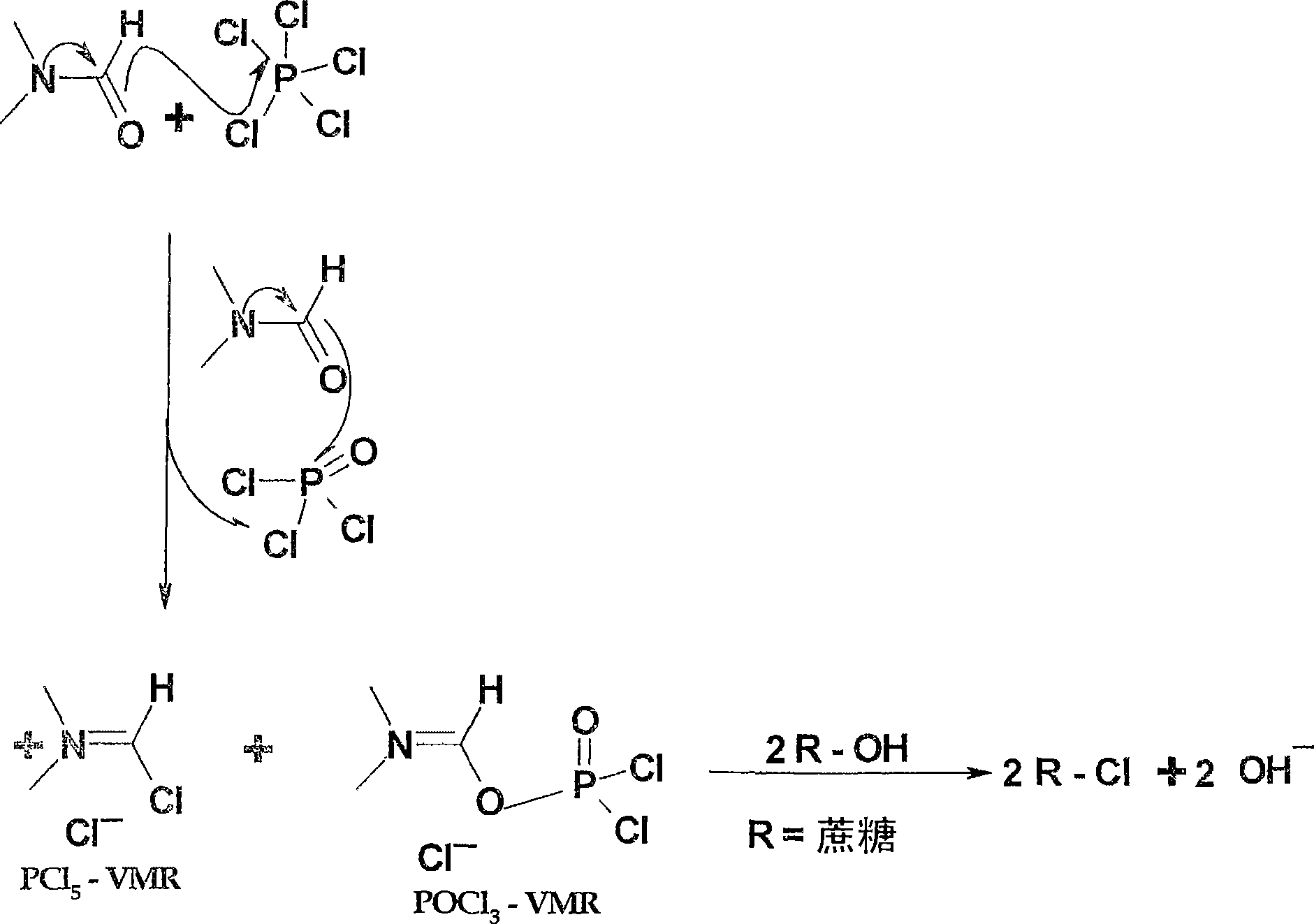
Generation of phosphorus oxychloride as by-product from phosphorus pentachloride and dmf and its use for chlorination reaction by converting into vilsmeier-haack reagent.
A technology for by-products and reagents, which is used in the production of by-product phosphorus oxychloride from phosphorus pentachloride and DMF and its application in the chlorination reaction by converting it into VILSMEIER-HAACK reagent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, after forming the first batch of Vilsmeier-Haack reagents, using 5 By-product POCl formed 3 Formation of the second batch of Vilsmeier-Haack reagents
[0034] At 20°C, 835g of PCl 5 Add to a round bottom flask containing 0.835L DMF. The completion of the Vilsmeier-Haack reaction was indicated by the formation of white crystals of the Vilsmeier-Haack reagent. After about 15 minutes, the generated POCl 3 Vilsmeier-Haack reagent also started to form and an orange-red solution formed with the solid. The mixture was then stirred thoroughly at room temperature for 1.0 hour. A 500ml excess of DMF was added to the reaction. The mixture was cooled to 0°C and a substrate containing 263 g of sucrose equivalent (sucrose-6-acetate) was added dropwise. The temperature was kept below 0°C during the dropwise addition.
[0035] After the substrate addition was complete, the temperature was allowed to come to room temperature and stirred for 1.0 hour. The temperature ...
Embodiment 2
[0036] Embodiment 2, utilize only by PCl 5 Chlorination of the formed Vilsmeier-Haack reagent
[0037] This experiment was performed to show that using only PCl 5 Efficiency of the chlorination reaction of the resulting Vilsmeier-Haack reagent. At 20°C, 835g of PCl 5 Add to a round bottom flask containing 0.835L DMF. The Vilsmeier-Haack reaction was complete and the formation of white crystals of the Vilsmeier-Haack reagent was observed. The reaction simultaneously forms POCl 3 , the POCl 3 Start the reaction with excess DMF to form the second Vilsmeier-Haack reagent. But the formed Vilsmeier-Haack reagent is in liquid form and does not become as in PCl 5 The solid Vilsmeier-Haack reagent in the reaction. Therefore, in order to determine and prove that by PCl 5 Efficiency of the formed Vilsmeier-Haack reagent, filtering off the formed PCl 5 Vilsmeier-Haack reagent, POCl 3 and excess DMF were thoroughly separated. The solid form of the Vilsmeier-Haack reagent was wa...
Embodiment 3
[0040] Embodiment 3, utilize only by POCl 3 Chlorination of the formed Vilsmeier-Haack reagent
[0041] This experiment was performed to show that using only POCl 3 Efficiency of the chlorination reaction of the resulting Vilsmeier-Haack reagent. 614.2g POCl 3 Add dropwise to a reaction flask containing 1250ml DMF. Keep the temperature at 0-5°C. Formation of the Vilsmeier-Haack reagent was determined by the formation of an orange color in the flask. The mixture was stirred for 1 hour to complete reagent formation and then the reaction was cooled to -5-0°C. A substrate containing 132 g of sucrose equivalent (sucrose-6-acetate) was added dropwise. The temperature was kept below 0°C during the dropwise addition.
[0042] After the substrate addition was complete, the temperature was allowed to come to room temperature and stirred for 1.0 hour. The temperature was then raised to 65°C and held for 1.5 hours and further heated to 80°C and held for 1.0 hour. The temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com