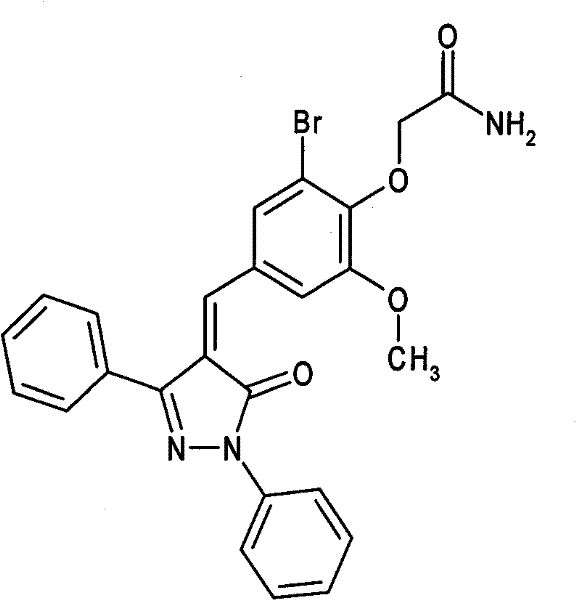
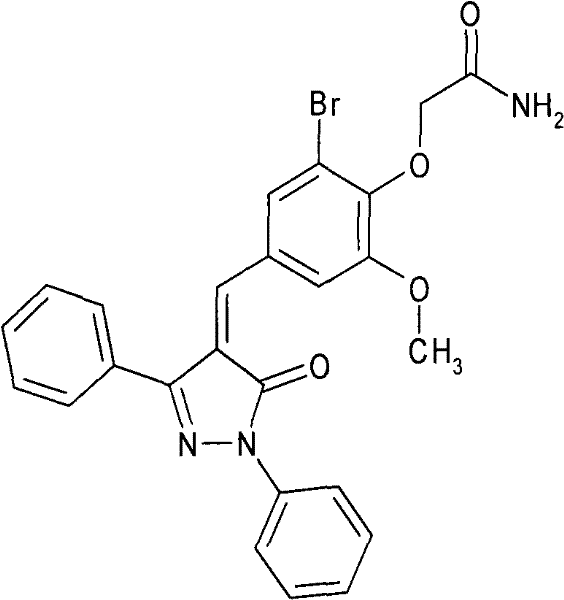
Application of 5-oxo-4-alkenylene-pyrazole derivative in preparing anti-tumor medicament
A technology of pyrazole derivatives and alkenylene, which is applied in the field of genetic engineering and chemistry to achieve obvious effects of tumor suppression, reduction of damage to normal cells, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1MTS method measures the growth inhibitory effect of FD10 on SK-Hep1 cells
[0042] Human liver cancer cell line SK-Hep1 cells (ATCC company) 2~5×10 3 Each well was inoculated into a 96-well plate, cultured for 24 hours to allow it to adhere to the wall, and then FD10 (Interchim, UZI / 9516006) was added to set up 6 concentration gradients and 3 replicate wells for each concentration. Cells at 37°C, 5% CO 2 After culturing for 72 hours under the same conditions, discard the culture medium, and use the MTS kit (Promega Company) to measure the cell survival rate. The test method is as follows: wash the cells once with serum-free medium, and add the pre-prepared MTS chromogenic solution (add 2 ml of solution 1 and 100 μl of solution 2 to 10 ml of serum-free medium, and mix thoroughly). A well without cells was set as the background well to correct for the background light absorption of the solution. Put the cells into the cell incubator and continue to culture ...
Embodiment 2
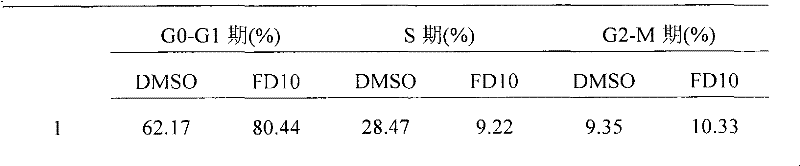
[0045] Effect of embodiment 2FD10 on cell cycle
[0046] Human liver cancer cell lines HepG2 cells (ATCC Company), 7721 cells (ATCC Company) and SK-CYPJ stable cell lines (constructed and preserved in our laboratory) 3×10 5 Inoculate in a 60mm cell culture dish per dish, and add FD10 after culturing for 24 hours to make it adhere to the wall. 37°C CO 2 The incubator was cultivated for 48hr. Collect the cell culture medium and cells together into a cytocentrifuge tube, centrifuge at 1000 rpm for 10 min, and discard the supernatant. The cells were washed once with 2ml of pre-cooled 1×PBS, centrifuged at 1000rpm for 10min, and the supernatant was discarded. Fix with 70% pre-cooled ethanol at 4°C for 2hr or longer (overnight is also acceptable). Centrifuge at 1000rpm for 10min, and carefully suck off the supernatant. Resuspended with 500-1000μl PI staining solution, stained in the dark at room temperature for 20min, filtered the membrane, and detected the cell cycle changes by...
Embodiment 3
[0056] Effect of embodiment 3FD10 on clone formation rate
[0057] 1000 cells / dish of SK-Hep1 cells (ATCC Company) were inoculated on 60 mm cell culture dishes, one group was added with FD10, and the other group was added with DMSO as a control. Continuously cultivate for 15-20 days, and change the medium as appropriate in the middle. Wash once with room temperature 1×PBS, add 2ml of pre-cooled methanol and fix at 4°C for 10min. Discard the methanol used for fixation, wash once with pre-cooled 1×PBS, add GIMESA stain, stain at room temperature for 10 min, wash 3 times with water, remove floating color, take pictures, and count.
[0058] The clone size is determined according to the number of cells. Those larger than 200 cells are called large clones, those between 100-200 cells are called medium clones, and those between 50-100 cells are called small clones. Comparing the difference between large and medium clones, repeating 2 times and taking the average of three dishes eac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com