Methods for administering long-lasting hypoglycemic agents
A technology of incretin and plasma, which is applied in the field of administering long-acting hypoglycemic drugs, and can solve the problem that therapeutic treatment of diabetes is not feasible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
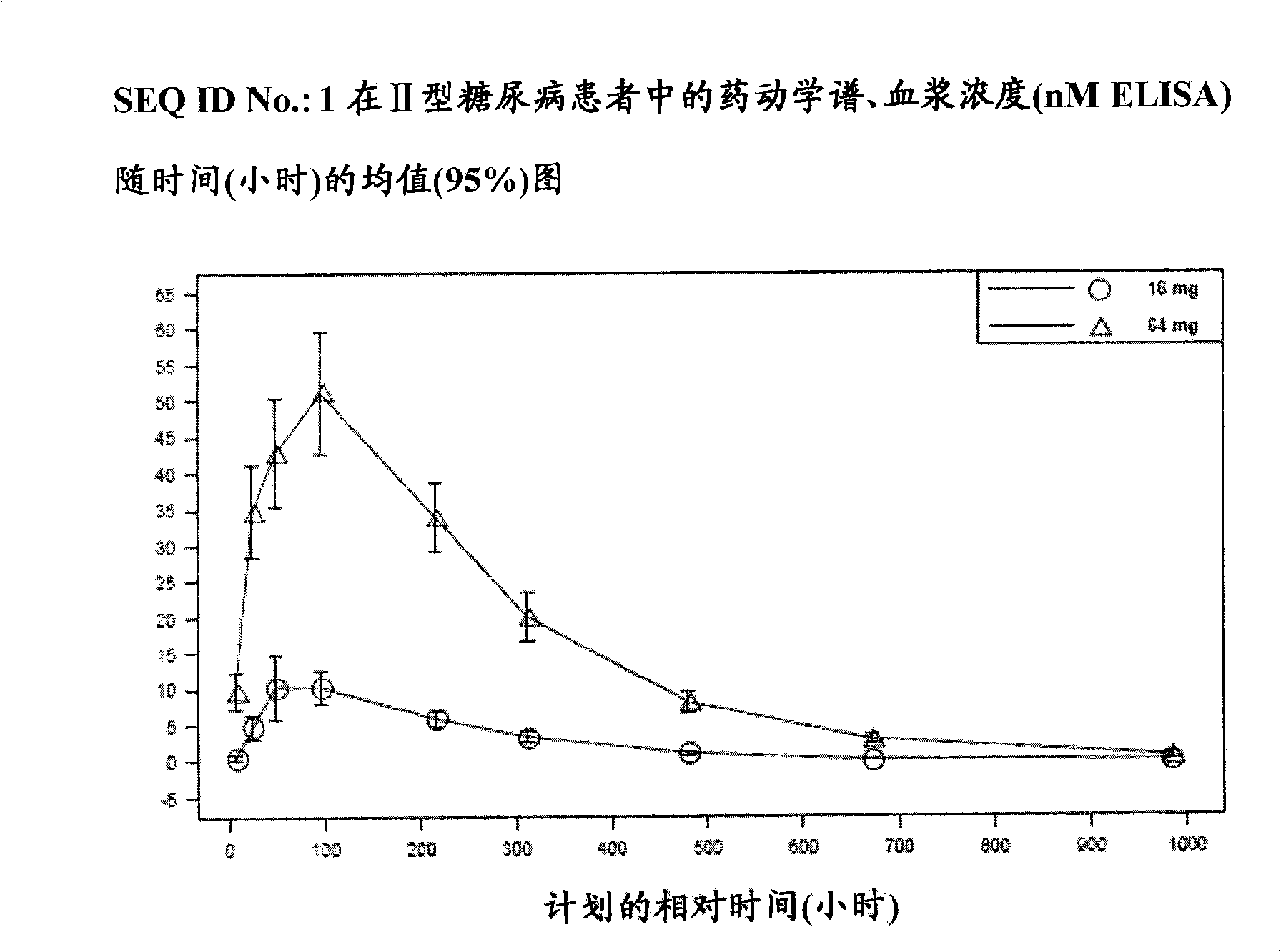
[0095]This was a single-blind, placebo-controlled dose escalation study in which a pharmaceutical composition comprising SEQ ID NO: 1 (0.25 mg-104 mg) was administered subcutaneously to the abdomen of healthy subjects.
[0096] Thirty-nine healthy male and female subjects participated in the study. Five groups of healthy subjects received the following two doses of the pharmaceutical composition comprising SEQ ID NO: 1 once a week by intraperitoneal subcutaneous injection: Group 1 (0.25 mg+1 mg); Group 2 (3 mg+6 mg ); Group 3 (16mg+24mg); Group 4 (48mg+60mg); and Group 5 (80mg+104mg). In Groups 1-4, 6 subjects were randomized to receive active compound and 2 subjects were randomized to receive placebo. In Group 5, 5 subjects were randomized to receive active compound and 2 subjects were randomized to receive placebo. Thus, 29 subjects received active treatment and 10 subjects received placebo. Exposure to the pharmaceutical composition comprising SEQ ID NO: 1 increased in a...
Embodiment 2
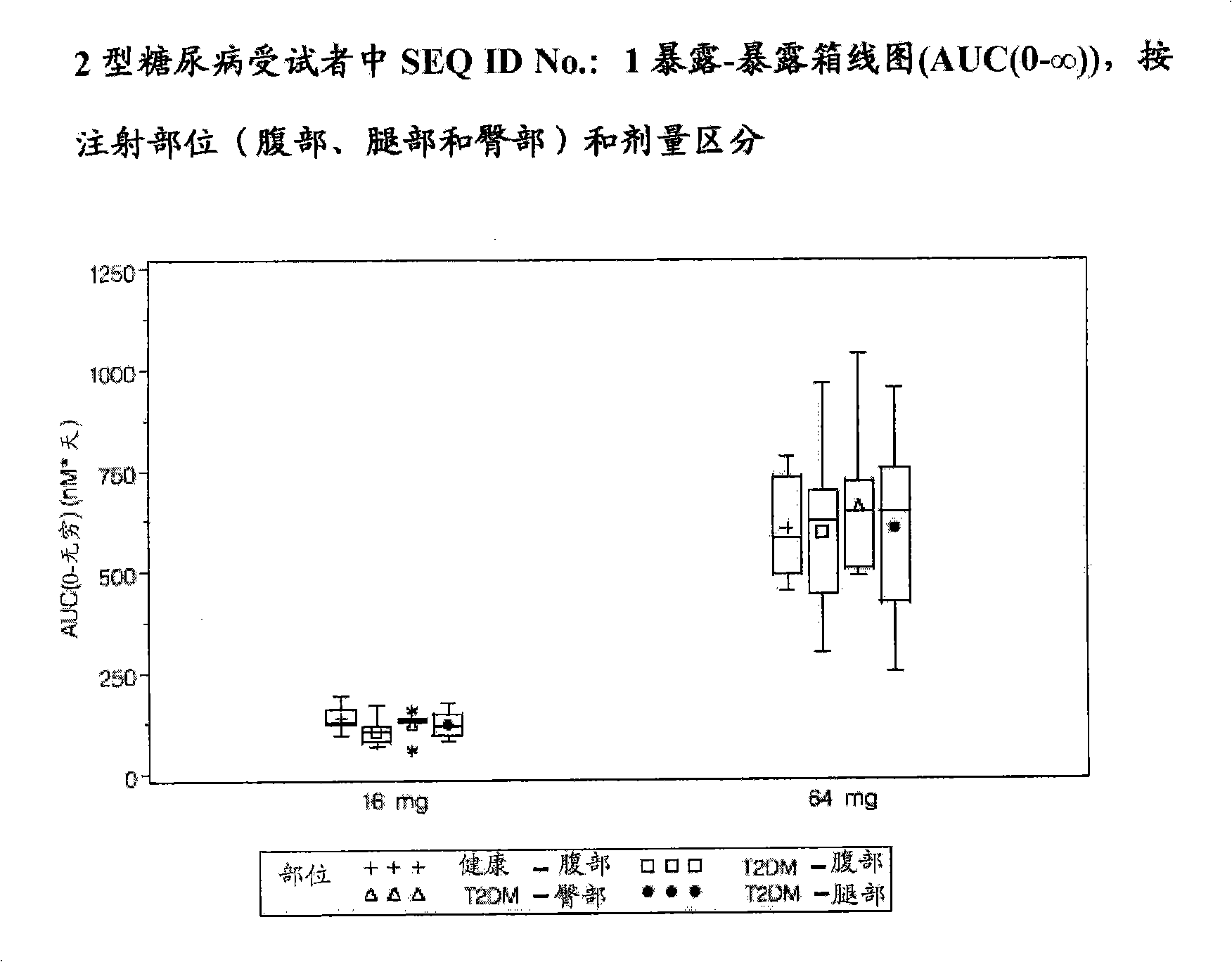
[0100] This was a single-blind, placebo-controlled, multiple-dose study in subjects with T2DM. Fifty-four male and female subjects participated in the study. Participants were either on a diet or taking metformin, a sulfonylurea, or a combination of metformin and a sulfonylurea. Subjects taking metformin and / or sulfonylureas prior to the start of the study were excluded from these treatments 2 weeks prior to the first dose. Subjects were randomized to receive placebo or a pharmaceutical composition comprising SEQ ID NO: 1 by intraperitoneal subcutaneous injection once a week for two weeks as follows: Group 1 (9 mg+9 mg; 4 placebo, 14 active) ; Group 2 (16mg+16mg; 5 placebo, 12 active); Group 3 (32mg+32mg; 5 placebo, 14 active). Forty subjects were randomized to active treatment and 14 subjects were randomized to placebo.
[0101] Fifty-three subjects completed the study. One subject in Cohort 2 who was randomized to receive active treatment withdrew from the study before t...
Embodiment 3
[0105] The pharmacodynamic profiles of the subjects described in Example 2 are provided in this Example. The subjects received subcutaneous injections of placebo or a pharmaceutical composition comprising SEQ ID NO: 1 once a week for two weeks as follows: Group 1 (9 mg+9 mg; 4 placebos, 14 actives); Group 2 (16mg+16mg; 5 placebo, 12 active); Group 3 (32mg+32mg; 5 placebo, 14 active). Subjects were dosed on Days 1 and 8. The effect of SEQ ID NO: 1 on glucose and fructosamine in T2DM subjects was evaluated. Subjects with T2DM were either on a controlled diet or taking metformin, a sulfonylurea, or a combination of metformin and a sulfonylurea, and had given up these treatments from 2 weeks before the first dose. Fasting and 24-hour glucose profiles were assessed at baseline and 24 hours after the first and second doses. Fructosamine was assessed at baseline and at follow-up visits (day 56 or 63) after day 3, day 21 and the last dose.
[0106] All subjects who received a prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com