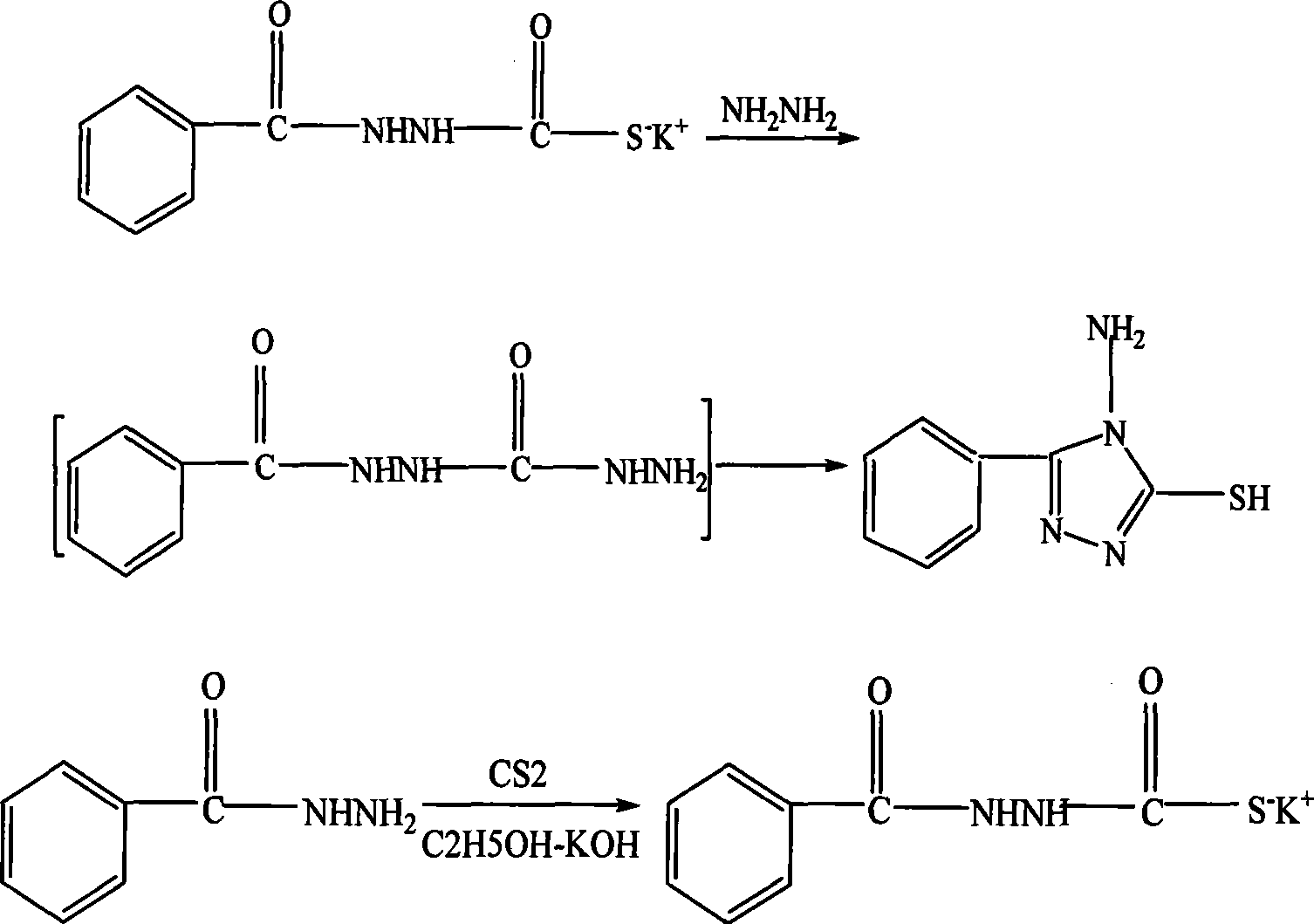
Method for preparing 5-phenyl-4-amino-triazolinthione
A technology of triazolethione and amino, which is applied in the field of preparation of nitrogen-containing organic corrosion inhibitors, can solve the problems of long reaction time, low synthesis yield, and many steps, and achieve shortened reaction time, optimized preparation conditions, and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 0.2 mol of benzoic acid into the reaction system containing 50 ml of absolute ethanol and 8 ml of concentrated sulfuric acid, reflux at 90° C. to 100° C. for 90 minutes, and distill off unreacted ethanol. Then it was cooled to room temperature, and Na 2 CO 3 solution until the gas stops escaping, collect the organic layer, and use anhydrous CaCl 2 Dry to obtain crude ethyl benzoate. Add the obtained crude ethyl benzoate to 0.35mol 85% hydrazine hydrate and 30ml absolute ethanol, react at 90°C-100°C for 3 hours, cool to room temperature, and suction-filter the precipitated phenylhydrazide crystals. Add phenylhydrazide crystals to 50ml of absolute ethanol dissolved with 0.24mol KOH, stir and drop into 0.20mol CS 2 React at room temperature for 16 hours, add anhydrous ether and filter with suction to obtain the potassium salt. Weigh 0.05mol of the above potassium salt, add it to 0.16mol of 85% hydrazine hydrate, react at 90°C to 100°C for 4 hours, pour it into 200m...
Embodiment 2
[0019] Add 0.2 mol of benzoic acid into the reaction system containing 50 ml of absolute ethanol and 8 ml of concentrated sulfuric acid, reflux at 90° C. to 100° C. for 60 minutes, and distill off unreacted ethanol. Then it was cooled to room temperature, and Na 2 CO 3 solution until the gas stops escaping, collect the organic layer, and use anhydrous CaCl 2 Dry to obtain crude ethyl benzoate. Add the obtained crude ethyl benzoate to 0.35mol 85% hydrazine hydrate and 30ml absolute ethanol, react at 90°C to 100°C for 2.5 hours, cool to room temperature, and filter the precipitated phenylhydrazide crystals with suction. Add phenylhydrazide crystals to 50ml of absolute ethanol dissolved with 0.24mol KOH, stir and drop into 0.20mol CS 2 React at room temperature for 16 hours, add anhydrous ether and filter with suction to obtain the potassium salt. Weigh 0.05 mol of the above potassium salt, add it to 0.16 mol of 85% hydrazine hydrate, react at 90°C to 100°C for 4 hours, pour ...
Embodiment 3
[0021] Add 0.2 mol of benzoic acid into the reaction system containing 50 ml of absolute ethanol and 8 ml of concentrated sulfuric acid, reflux at 90° C. to 100° C. for 75 minutes, and distill off unreacted ethanol. Then it was cooled to room temperature, and Na 2 CO 3 solution until the gas stops escaping, collect the organic layer, and use anhydrous CaCl 2 Dry to obtain crude ethyl benzoate. Add the obtained crude ethyl benzoate to 0.35mol 85% hydrazine hydrate and 30ml absolute ethanol, react at 90°C-100°C for 3.5 hours, cool to room temperature, and suction-filter the precipitated phenylhydrazide crystals. Add phenylhydrazide crystals to 50ml of absolute ethanol dissolved with 0.50mol KOH, stir and drop into 0.20mol CS 2 React at room temperature for 16 hours, add anhydrous ether and filter with suction to obtain the potassium salt. Weigh 0.05mol of the above potassium salt, add it to 0.16mol of 85% hydrazine hydrate, react at 90°C to 100°C for 3 hours, pour into 200ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com