New method for detecting plague antibodies in serum sample and product thereof
A technology for detecting antibodies and plague, applied to measuring devices, instruments, fluorescence/phosphorescence, etc., can solve the problems of lack of models and evaluations, and achieve good consistency results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] 2. Preparation of samples to be tested
[0046] 1. Preparation of target analyte samples
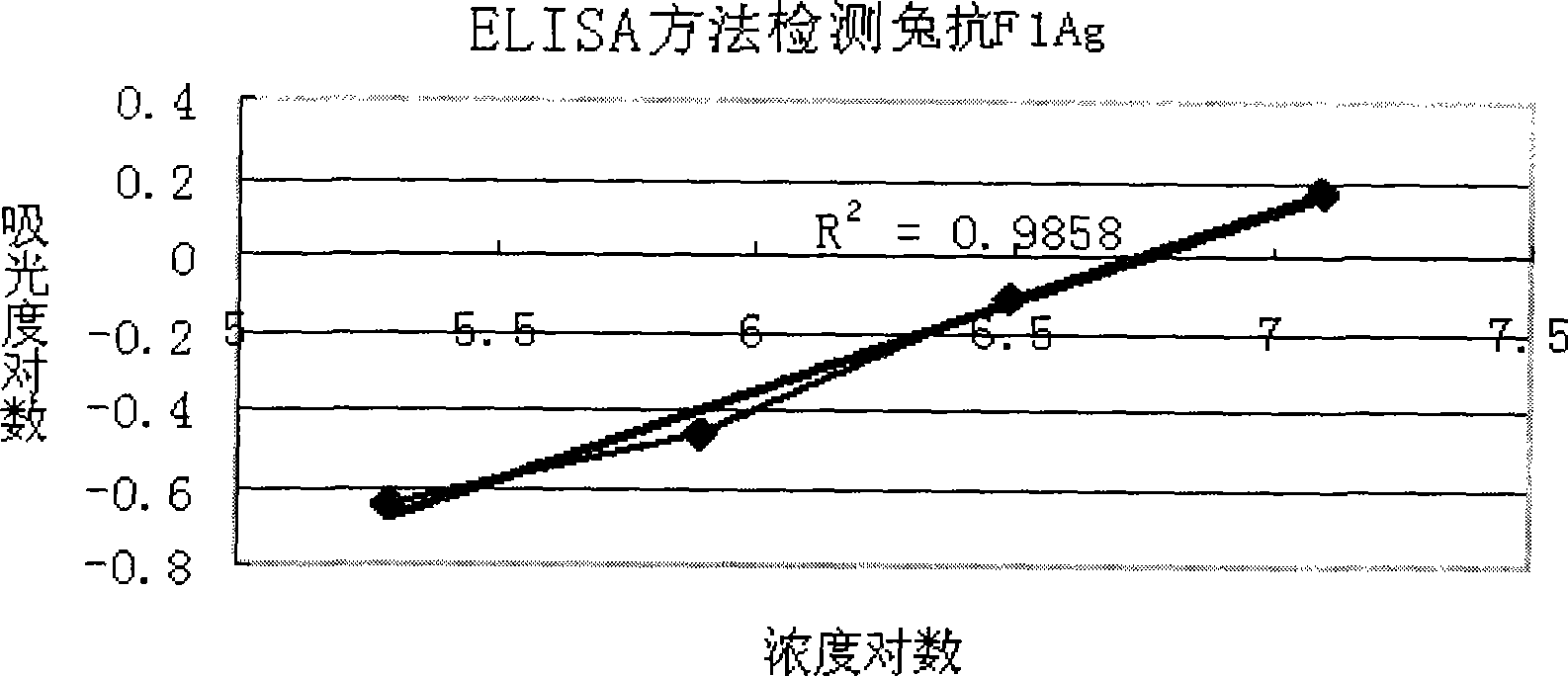
[0047] The target analyte is rabbit anti-plague F1 IgG, the interfering sample or the sample used as method-specific test is other antibodies or other proteins other than the target detection object, including rabbit anti-tuberculosis serum, rabbit anti-SARS IgG, rabbit anti-avian influenza H5N1 serum, rabbit Anti-plague IgG, BSA, casein, tryptone, etc. All the above-mentioned samples to be analyzed were dissolved in the sample diluent and stored at 4°C. The stock solution concentration of rabbit anti-avian influenza H5N1 IgG is 0.2mg / mL. In the comparison experiment, the same sample was used for the detection of ELISA and suspension chip.
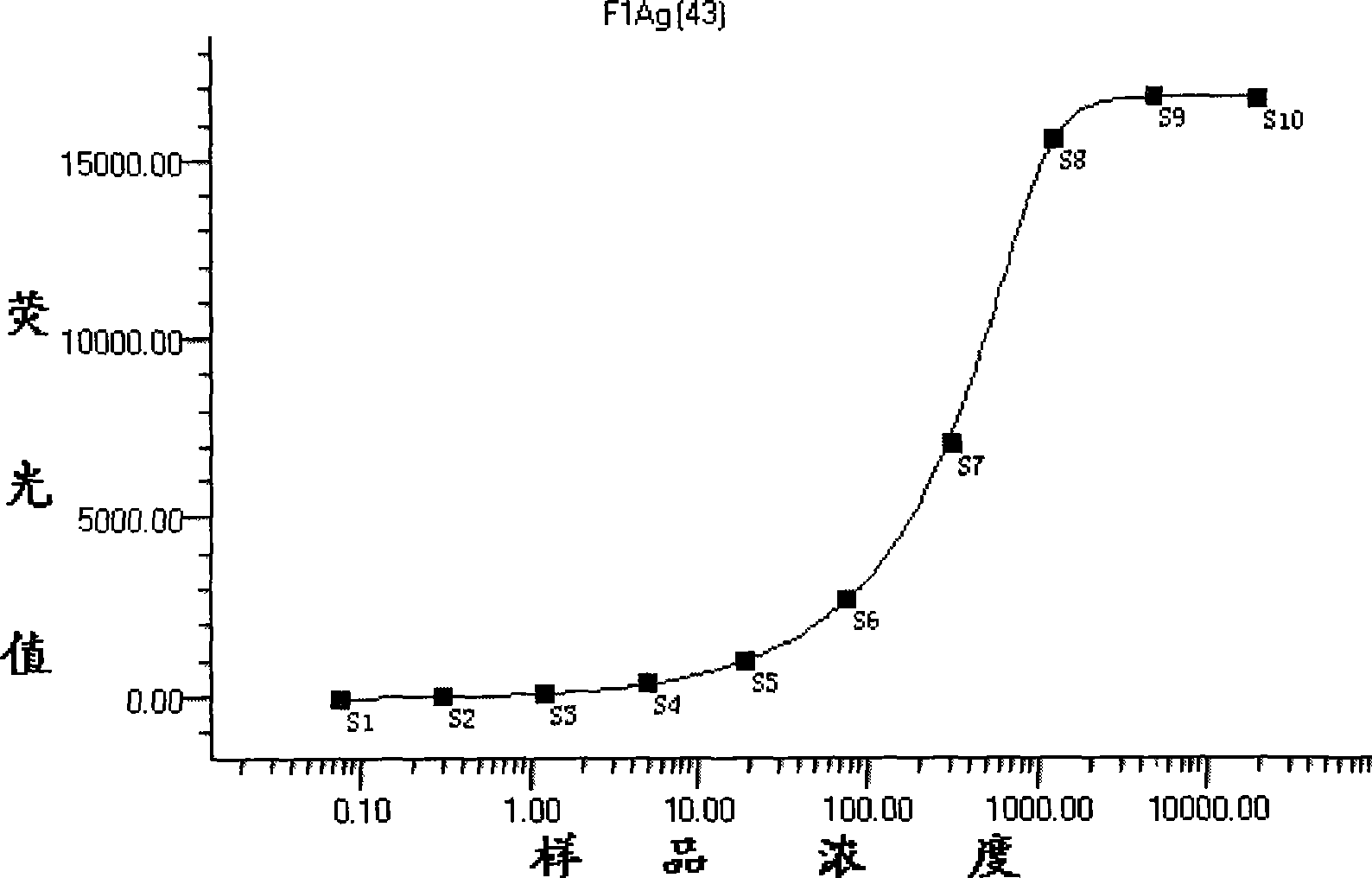
[0048] Dilute the rabbit anti-plague F1 IgG sample diluent to be analyzed into samples of different concentrations in a 4-fold ratio to draw a standard curve for sample detection dose-response. Among them, the concentration of several sampl...
Embodiment 1
[0051] Example 1. Preparation of a protein suspension chip for detecting plague antibodies
[0052] 1. Capturing antigen-coated encoded microspheres
[0053] The No. 043 coded microsphere used in the present invention was purchased from BIO-RAD Company of the United States. The coded microsphere was used to label the plague F1 protein antigen capable of capturing plague antibodies, that is, the plague F1 protein was used to coat the microsphere.
[0054] A. Activation of encoded microspheres
[0055] Take 100 μL (1.25×106) encoded microspheres into a 1.5mL centrifuge tube, centrifuge at 14000×g, carefully aspirate and discard the supernatant. Add 100 μL of microsphere washing buffer to suspend, shake and sonicate, centrifuge at 14000×g, carefully aspirate and discard the supernatant. Add 100 μL of microsphere activation buffer, then add 10 μL of freshly prepared EDC (50 mg / mL), then add 10 μL of freshly prepared 50 mg / mL Sulfo-NHS, shake at high speed for 30 seconds, wrap in...
Embodiment 2
[0065] Embodiment 2, optimization of suspension chip preparation method conditions
[0066] 1. Selection of the amount of antigen coating on microspheres
[0067] 100 μL of microspheres coded as No. 027 were coated with 1 μg, 3 μg, 6 μg, 9 μg, 12 μg, 15 μg, and 20 μg, respectively. After testing the effect comparison, with 6μg / 1.25×10 6 A microsphere, that is, 12-24ng / 2500-5000 microspheres / test coating, has the best coating effect. After counting under a microscope, store it in a dark place and refrigerate it for later use.
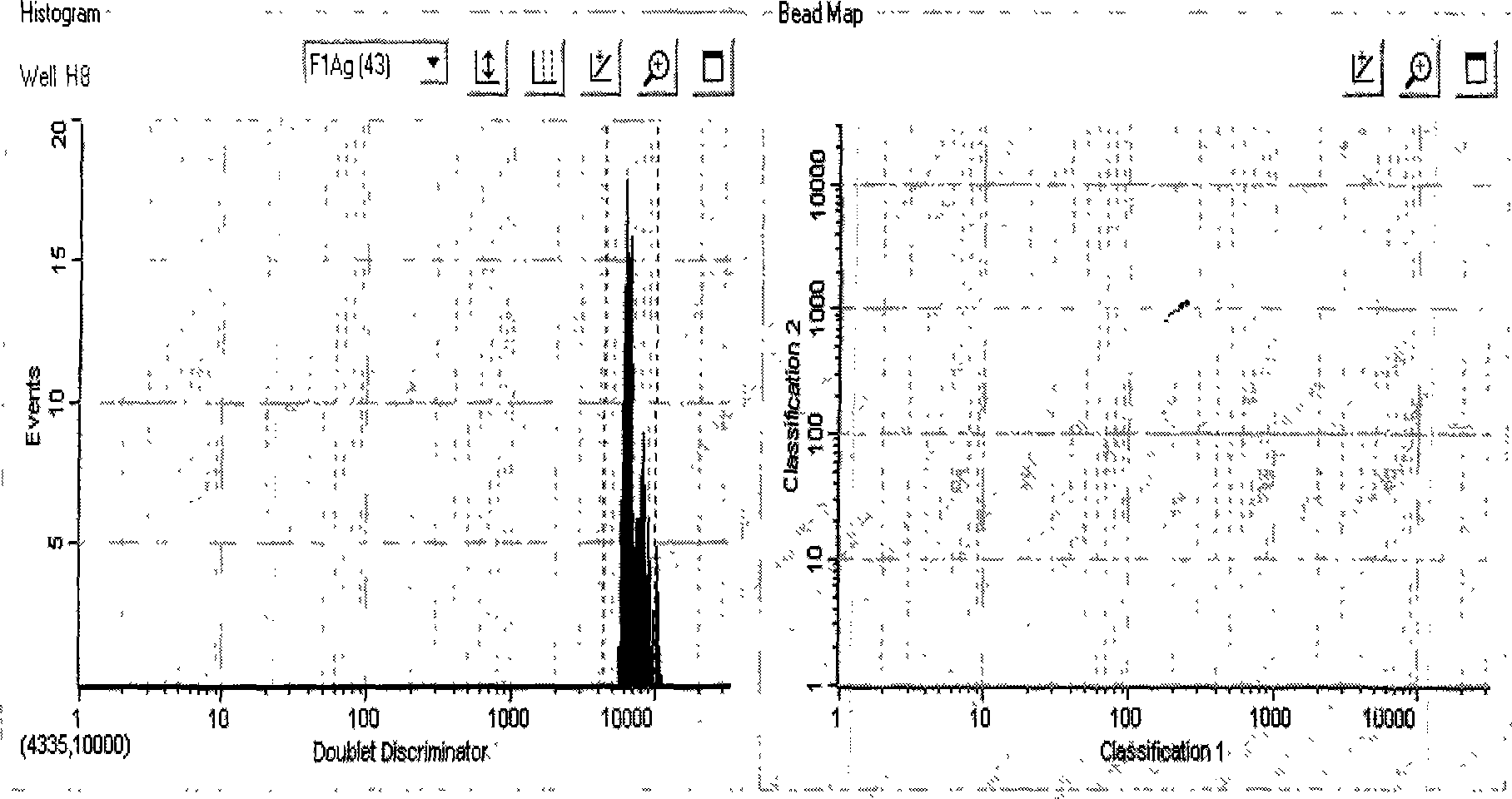
[0068] Such as figure 1 As shown, the No. 043 microspheres coated with plague F1 protein antigen all fell in the correct detection area, and obtained high signal-to-noise ratio results (MFI value is much greater than 2000), indicating that the optimized suspension chip detection system can be successfully used for plague Antibody detection.
[0069] 2. Optimization of biotinylated antibodies
[0070] The present invention respectively uses amino act...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com