Pharmaceutical composition for preventing or treating parkinsonism and preparation method thereof
A Parkinson's disease and composition technology, applied in the field of pharmaceutical compositions, can solve the problems of Parkinson's disease that have not yet been seen, and achieve the effects of prevention and treatment of Parkinson's disease, easy quality control, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
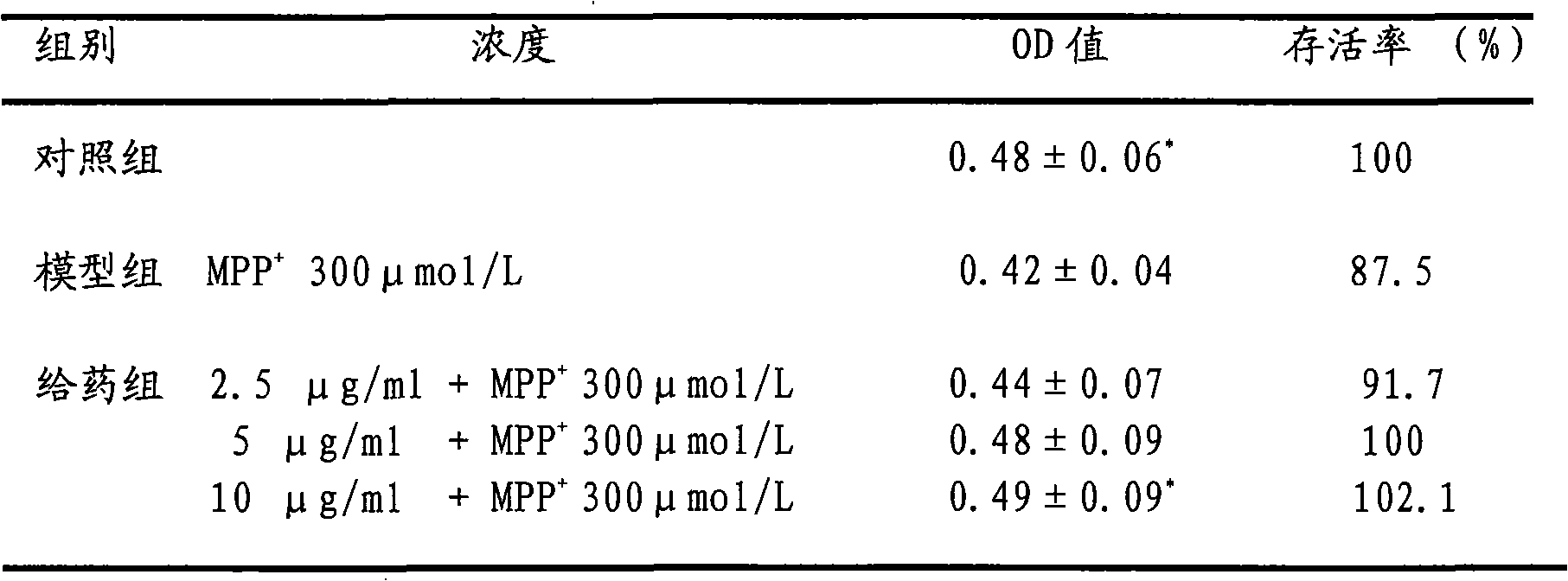
experiment example 1
[0018] 1. Experimental cell line: Shanghai Cell Institute, Chinese Academy of Sciences
[0019] 2. Experimental drug: MPP+ SIGMA-RBI company
[0020] Eleutheroside B standard
[0021] 3. Experimental method: make PC12 cells into a single cell suspension, and use 5×10 4 / ml inoculated in 96-well culture plate. After continuous culture for 24 hours, the medium was changed, and different concentrations of eleutheroside B dilutions were added to each well, so that the final concentrations were 2.5, 5, 10, 20, 40, and 80 μg / ml, and the final concentrations were added at the same time. 300μmol / L MPP + solution. After further culturing for 48 hours, 20 μl of MTT solution (5 mg / ml) was added to each well, and incubated at 37° C. for 4 hours. After terminating the culture, carefully suck out the medium, add 150 μl DMSO to each well, and shake for 10 minutes to fully dissolve the crystals. The absorbance value of each well at 490nm was detected by a microplate reader,...
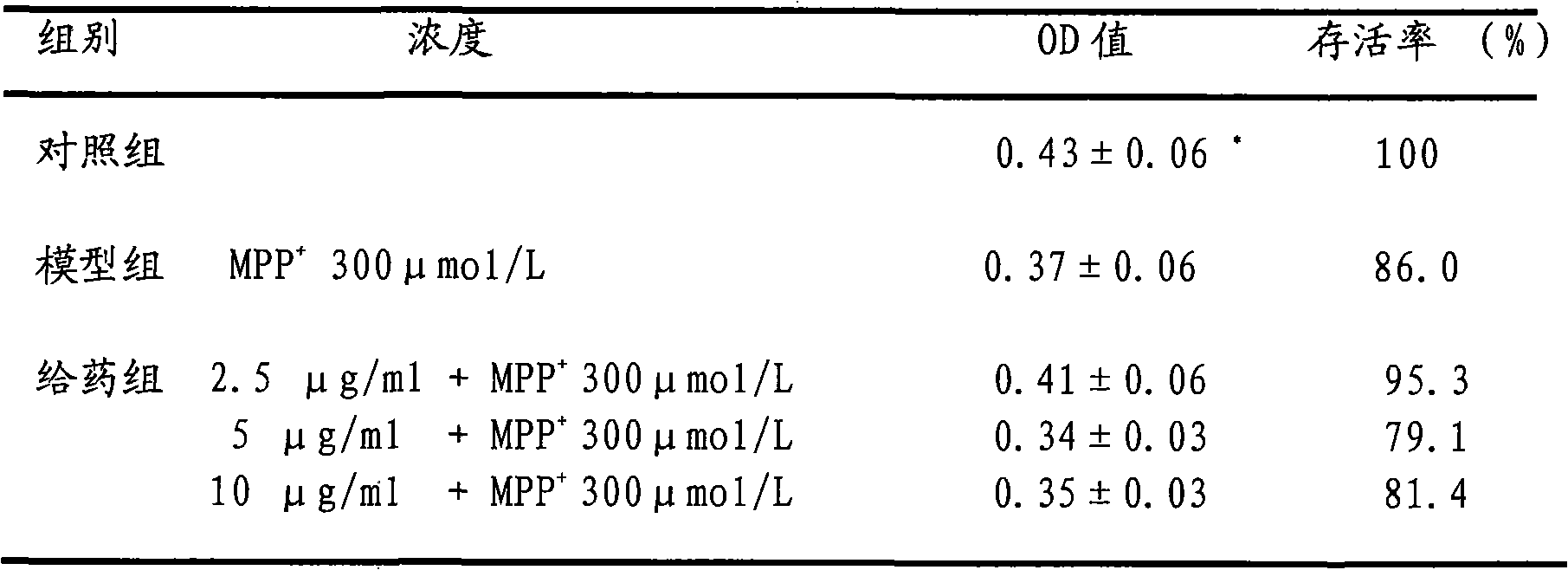
experiment example 2
[0030] 1. Experimental cell line: Shanghai Cell Institute, Chinese Academy of Sciences
[0031] 2. Experimental drug: MPP+ SIGMA-RBI company
[0032] Eleutheroside D standard
[0033] 3. Experimental method: make PC12 cells into a single cell suspension, and use 5×10 4 / ml inoculated in 96-well culture plate. After continuous culture for 24 hours, the medium was changed, and different concentrations of eleutheroside D dilutions were added to each well so that the final concentrations were 2.5, 5, 10, 20, 40, and 80 μg / ml, and the final concentrations were added at the same time. 300μmol / L MPP + solution. After further culturing for 48 hours, 20 μl of MTT solution (5 mg / ml) was added to each well, and incubated at 37° C. for 4 hours. After terminating the culture, carefully suck out the medium, add 150 μl DMSO to each well, and shake for 10 minutes to fully dissolve the crystals. The absorbance value of each well at 490nm was detected by a microplate reader, ...
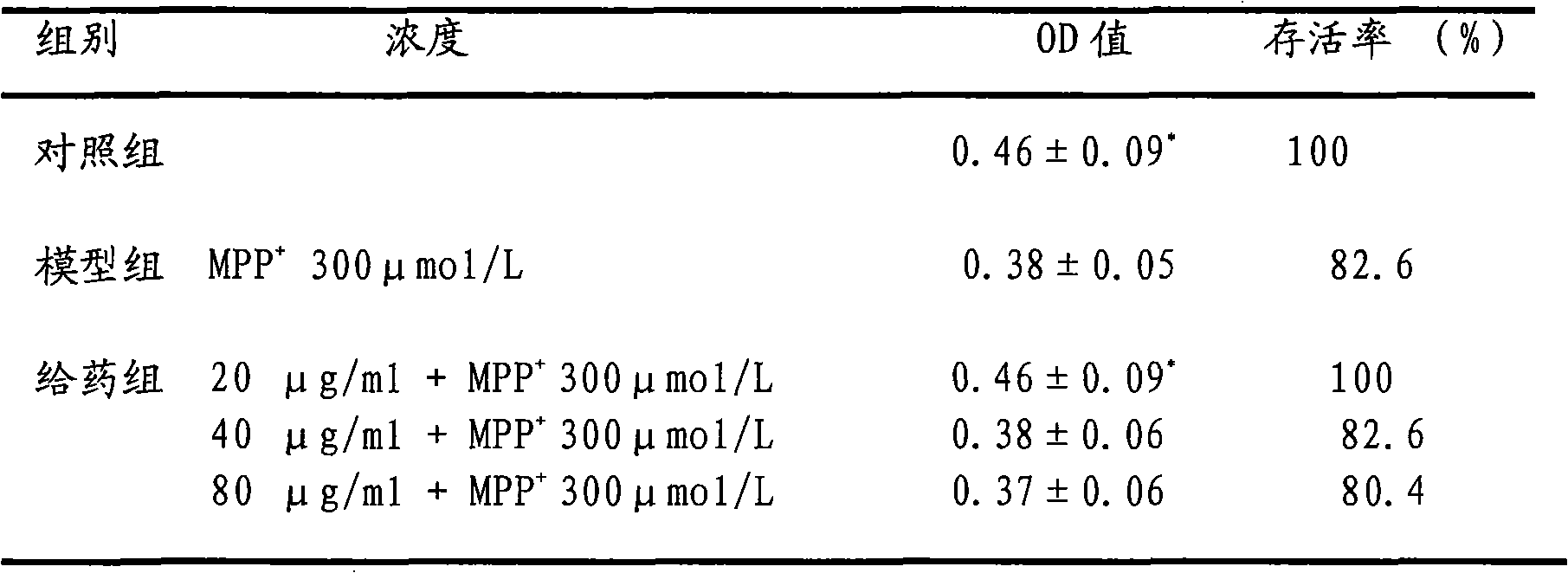
experiment example 3
[0043] 1. Experimental cell line: Shanghai Cell Institute, Chinese Academy of Sciences
[0044] 2. Experimental drug: MPP+ SIGMA-RBI company
[0045] Eugenol diglucoside
[0046] 3. Experimental method: make PC12 cells into a single cell suspension, and use 5×10 4 / ml inoculated in 96-well culture plate. After continuous culture for 24 hours, the medium was changed, and different concentrations of eugenol diglucoside dilutions were added to each well, so that the final concentrations were 2.5, 5, 10, 20, 40, and 80 μg / ml. Concentration 300μmol / L MPP + solution. After further culturing for 48 hours, 20 μl of MTT solution (5 mg / ml) was added to each well, and incubated at 37° C. for 4 hours. After terminating the culture, carefully suck out the medium, add 150 μl DMSO to each well, and shake for 10 minutes to fully dissolve the crystals. The absorbance value of each well at 490nm was detected by a microplate reader, and the cell viability was calculated. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com