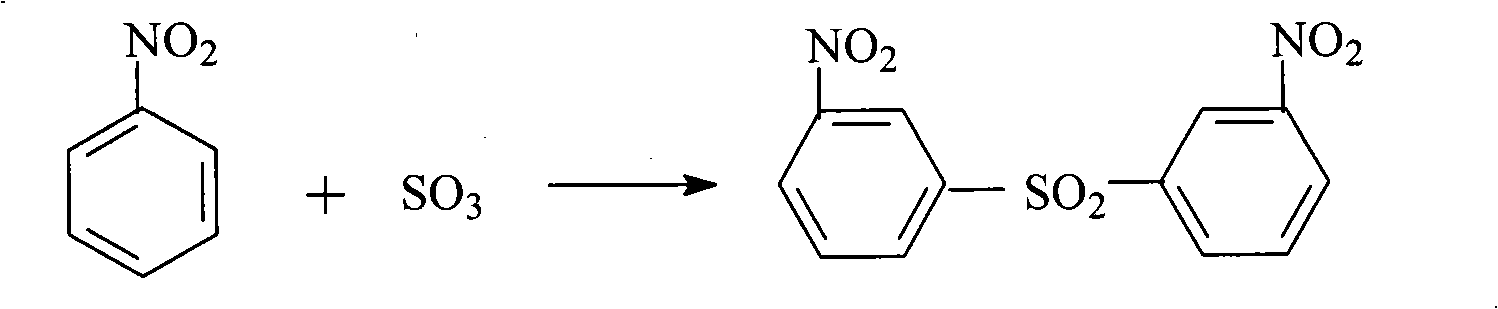
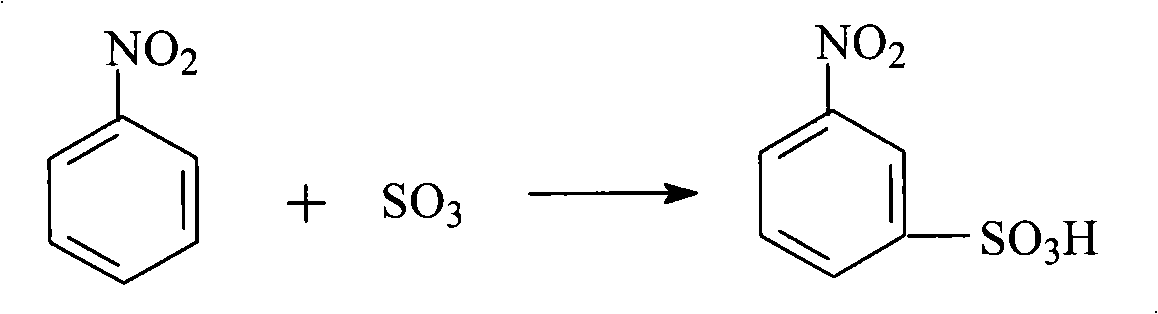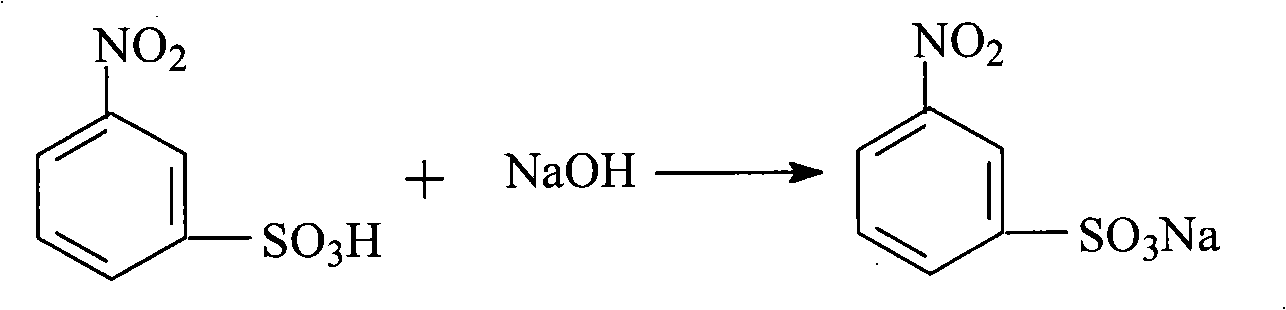Method for preparing m-aminophenol by catalytic hydrolysis of m-phenylenediamine
A technology of m-aminophenol and m-phenylenediamine, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxy compounds, etc., can solve problems such as no cost advantage, and achieve high product yield and good product quality. , the effect of low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 250ml autoclave made of zirconium, add 4.5g of m-phenylenediamine, 50ml of water, and 6.6ml of 37% hydrochloric acid, seal the autoclave, and replace it with nitrogen 2 to 3 times. Heat up to 205°C. At this time, the pressure in the kettle is 1.9 MPa. Keep the temperature for 4 hours, cool down to 30°C, release the pressure in the kettle, pour the contents of the kettle into a separatory funnel, and use 3*20ml methyl The isobutyl ketone was extracted three times to combine the organic phase, the organic phase was demethylated by methyl isobutyl ketone, and the resorcinol with a purity of 99% was obtained by vacuum distillation. The previous aqueous phase was adjusted to pH 14 with sodium hydroxide, extracted three times with 3*20ml methyl isobutyl ketone, the organic phase was combined, back-extracted with 20ml 5% hydrochloric acid, the organic phase was combined, and methyl isobutyl ketone was obtained after phase separation The ketone is reused to obtain the m-ph...
Embodiment 2
[0035] The basic preparation process is the same as in Example 1, the difference is that 4.5g of m-phenylenediamine, 50ml of water, and 6.6ml of 37% hydrochloric acid are respectively added to the autoclave made of zirconium, and the temperature is raised to 180°C. At this time, the pressure in the autoclave is 0.9MPa , keep the temperature for 10 hours, and cool down. The sample was analyzed by high-pressure liquid chromatography, and the conversion rate of m-phenylenediamine was 57%, and the selectivity of m-aminophenol was 91%.
Embodiment 3
[0037] The basic preparation process is the same as in Example 1, the difference is that 4.5g of m-phenylenediamine, 50ml of water, and 7.6ml of 37% hydrochloric acid are respectively added to the autoclave made of zirconium, and the temperature is raised to 190°C. At this time, the pressure in the autoclave is 1.2MPa , keep the temperature for 9 hours, and cool down. The sample was analyzed by high-pressure liquid chromatography, and the conversion rate of m-phenylenediamine was 51%, and the selectivity of m-aminophenol was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com