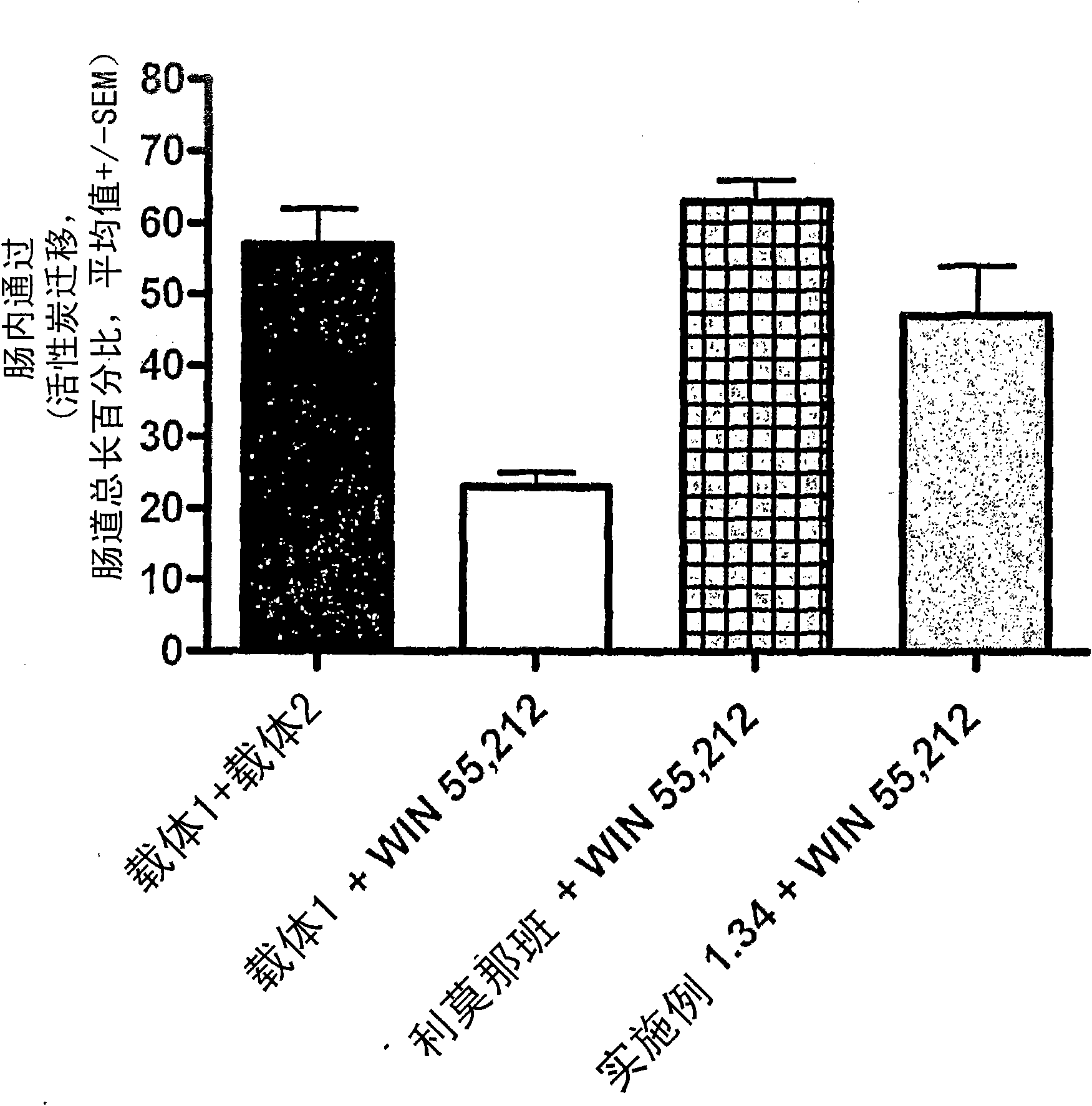Modulators of cb1 receptors
A C1-C4, R14 technology, applied in the field of compounds with normal signal transduction activity, to reduce the tendency to induce neurological and nervous system side effects, and to achieve low central effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
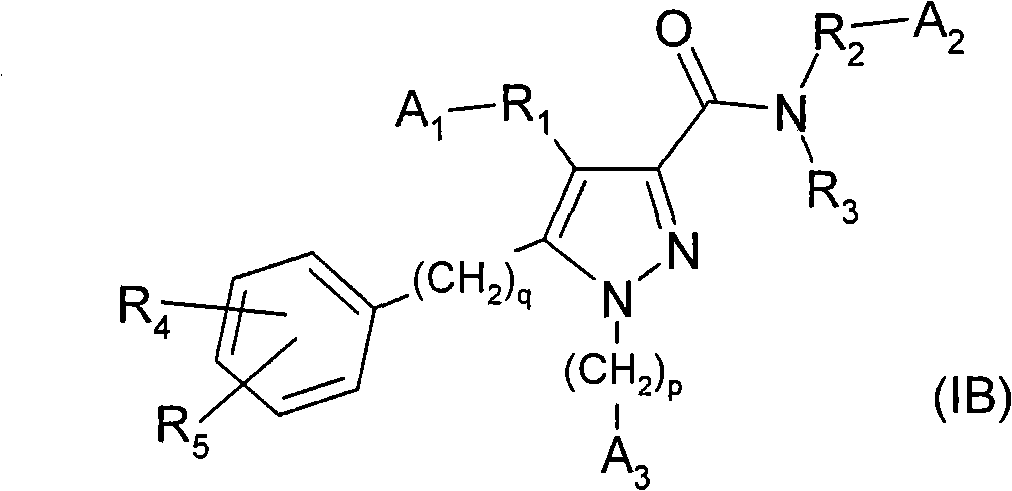
[0061] According to a first aspect of the present invention, there is provided a compound of formula (IB), or a salt, hydrate, solvate, single enantiomer or N-oxide thereof:
[0062]
[0063] in:
[0064] A 1 is hydrogen, -COOH, or tetrazolyl;
[0065] p and q are independently 0 or 1;
[0066] A 3 is phenyl or cycloalkyl, either optionally replaced by R 4 and / or R 5 replace;
[0067] R 4 and R 5 Standalone for -R 9 , -CN, -F, -Cl, -Br, -OR 9 , -NR 7 R 8 , -NR 7 COR 6 , -NR 7 SO 2 R 6 、-COR 6 、-SR 9 、-SOR 9 or -SO 2 R 6 ;
[0068] R 6 is C 1 -C 4 Alkyl, cycloalkyl, -CF 3 or -NR 7 R 8 ;
[0069] R 7 and R 8 independently for hydrogen, C 1 -C 4 Alkyl or cycloalkyl;
[0070] R 9 is hydrogen, C 1 -C 4 Alkyl, C 1 -C 4 Alkoxy, C 1 -C 4 Alkoxy (C 1 -C 4 Alkyl)-, cycloalkyl, fully or partially fluorinated C 1 -C 4 alkyl;
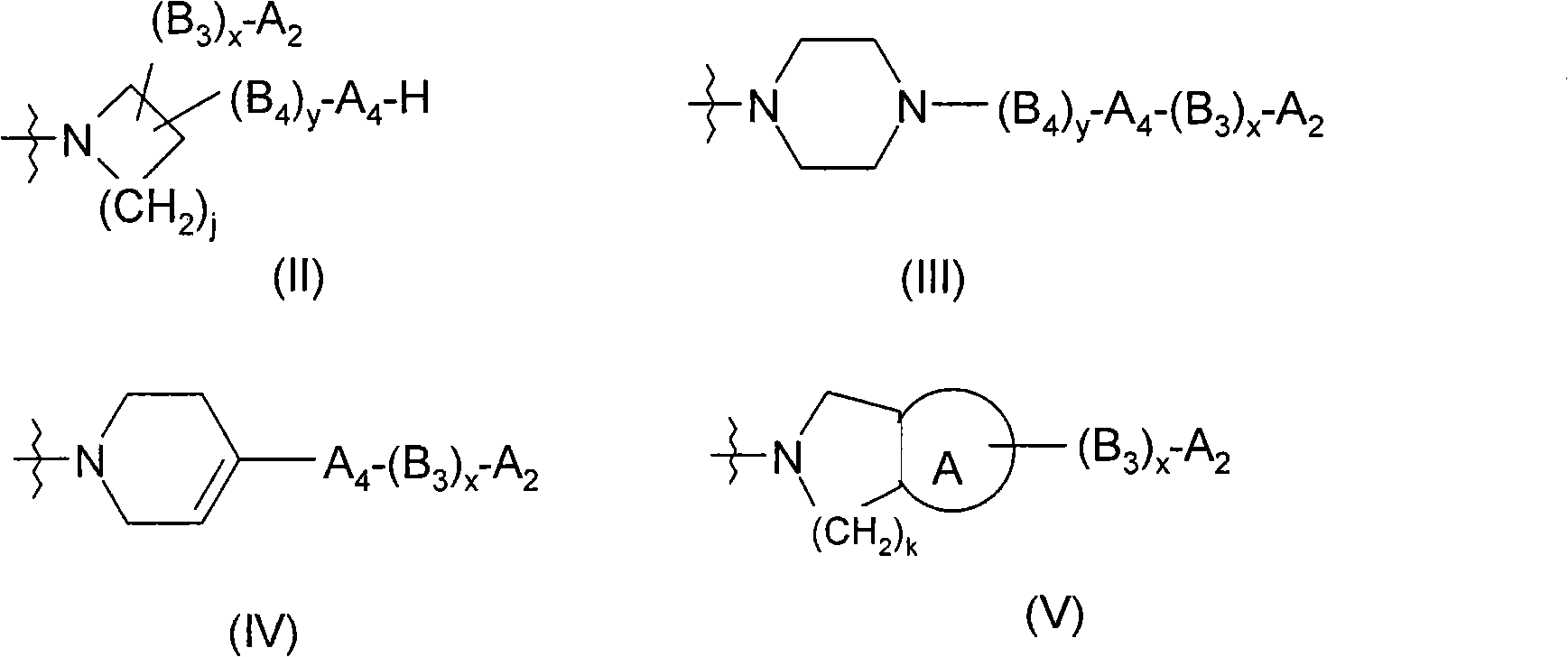
[0071] R 1 yes
[0072] (i) key, or
[0073] (ii)-(CH 2 ) a B 1 (CH 2 ) b -, where a and b are independentl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com