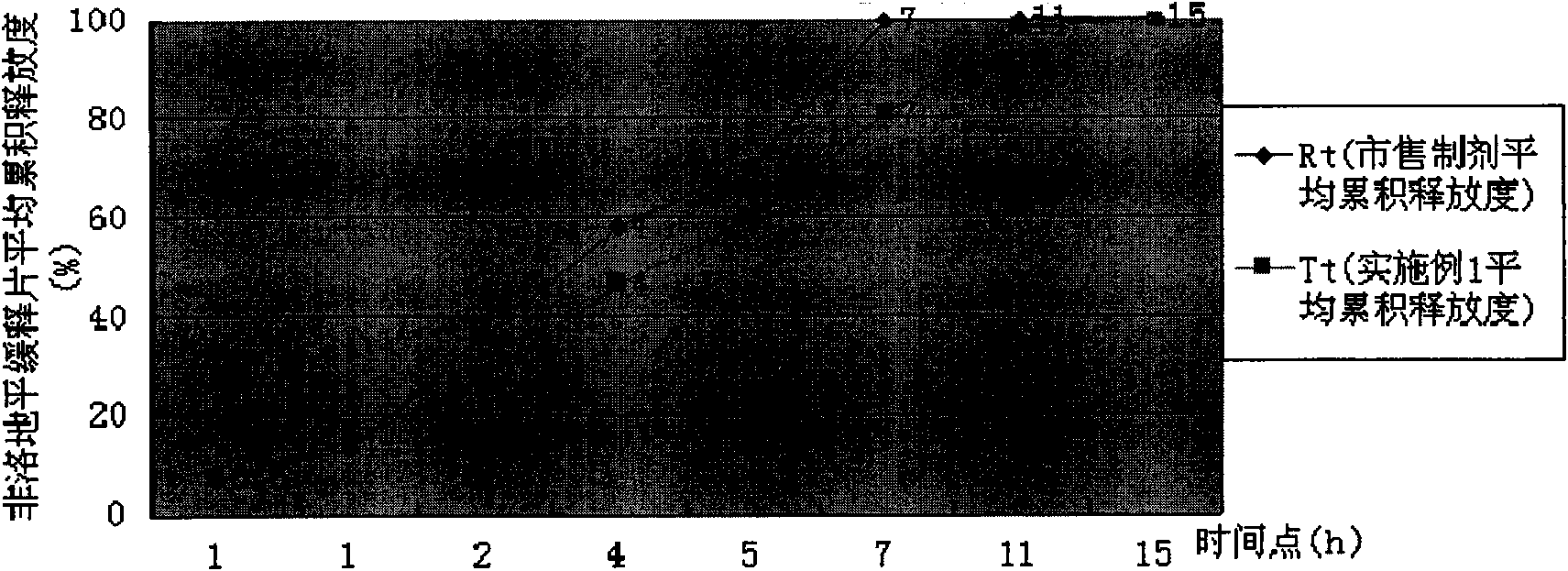Felodipine sustained-release tablet and method for controlling sustained-release of Felodipine sustained-release tablet
A gentle and filodipine technology, applied to non-active ingredient medical preparations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems such as retention and no release patents for felodipine slow-release controlled-release preparations , to achieve the effect of reducing rework, reducing energy consumption, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take by weighing Felodipine sustained release tablet granule 15.2g (wherein the total amount of HPMC K15M CR, HPMC K4M CR and HPMC K100LV CR accounts for 33% of granule gross weight; The weight ratio of the three is: HPMCK15M CR: HPMC K4M CR: HPMC K100LV CR=2:1:4), add 50g of hot distilled water, stir with a glass rod to disperse it, add 434.8g of distilled water, and fully stir for 40 minutes with a high-shear mixing emulsifier to prepare an aqueous solution containing 1% HPMC, using Brookfield LVDV -C digital display viscometer, choose #1 rotor, the measured viscosity under the condition of rotating speed 30rpm is 7040 centipoise. The release rate of felodipine sustained-release tablets prepared at this time is shown in Table 1, and the dissolution curve similarity f2 factor method evaluation is shown in Table 1. figure 1 .
[0034] The release degree of table 1 embodiment 1
[0035]
[0036] Conclusion: Example 1 is evaluated according to the dissolution ...
Embodiment 2
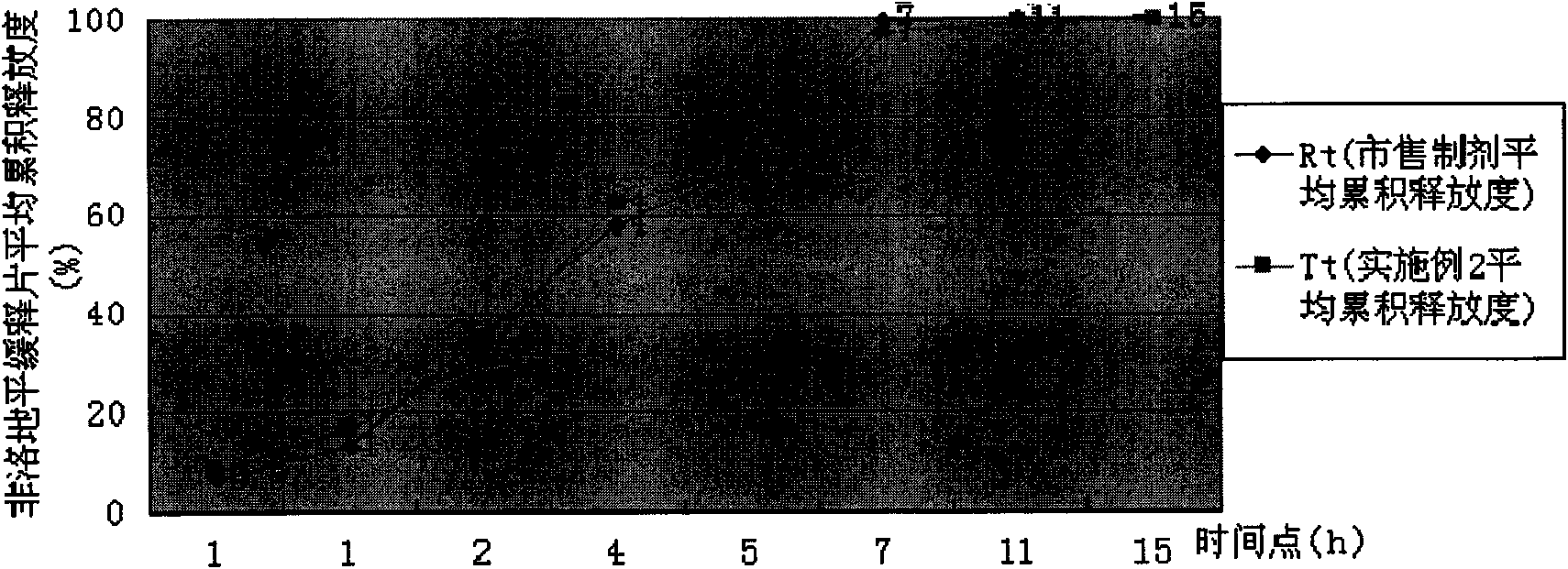
[0038] Take felodipine sustained-release tablet granule 12.1g (wherein the total amount of HPMC K4M CR and HPMC K100LVCR accounts for 41.4% of granule gross weight; The weight ratio of the two is: HPMC K4M CR: HPMCK100LV CR=4: 25), add 50g of hot distilled water, stirred with a glass rod to disperse, add 437.9g of distilled water, and fully stir for 40 minutes with a high-shear mixer emulsifier to prepare an aqueous solution containing 1% HPMC, using a Brookfield LVDV-C digital display viscometer, and select the #1 rotor , The measured viscosity under the condition of rotating speed 30rpm is 2820 centipoise. The release rate of felodipine sustained-release tablets prepared at this time is shown in Table 2, and the dissolution curve similarity f2 factor method evaluation is shown in Table 2. figure 2 .
[0039] The release degree of table 2 embodiment 2
[0040] time
[0041] Conclusion: Example 2 is evaluated according to the dissolution curve similarity f2 facto...
Embodiment 3
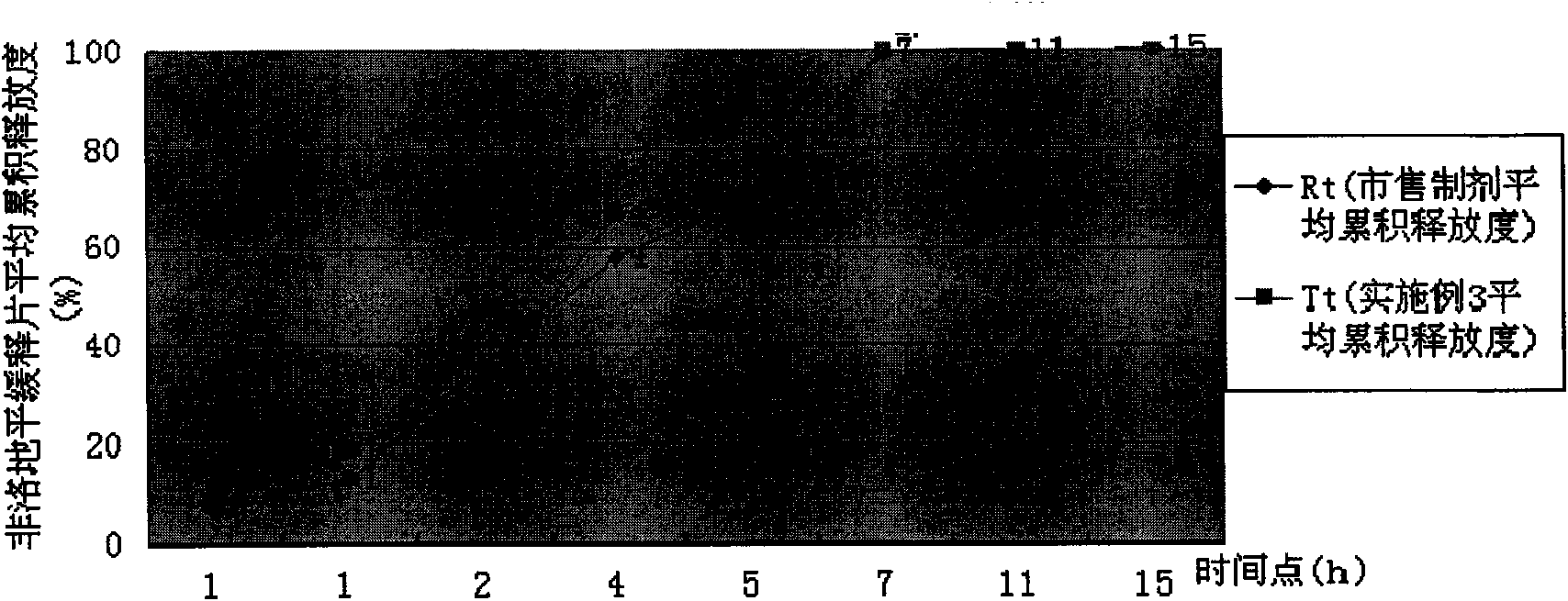
[0043] Take by weighing Felodipine sustained-release tablet granule 12.5g (wherein the total amount of HPMC K15M CR, HPMC K100LVCR and HPMC E15LV accounts for 40.1% of the granule gross weight; The weight ratio of the three is: HPMCK15M CR: HPMC K100LV CR: HPMC E15LV= 3:8:6), add 50g of hot distilled water, stir with a glass rod to disperse it, add 437.5g of distilled water, and fully stir for 40 minutes with a high-shear mixing emulsifier to prepare an aqueous solution containing 1% HPMC, using Brookfield LVDV-C Apparent viscometer, choose #1 rotor, the measured viscosity of rotating speed 30rpm condition is 2200 centipoises. The release rate of the felodipine sustained-release tablets prepared at this time is shown in Table 3, and the dissolution curve similarity f2 factor method evaluation is shown in Table 3. image 3 .
[0044] The release degree of table 3 embodiment 3
[0045]
[0046] Conclusion: Example 3 is evaluated according to the dissolution curve sim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com