Novel oral capsule preparation for treating cancer and AIDS
A technology of AIDS and capsules, which is applied in the field of new capsule oral preparations of polypeptide drugs, can solve the problems of large side effects and limit the clinical application of interleukin 2, and achieve the effects of improving penetration, increasing intestinal half-life, and increasing transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
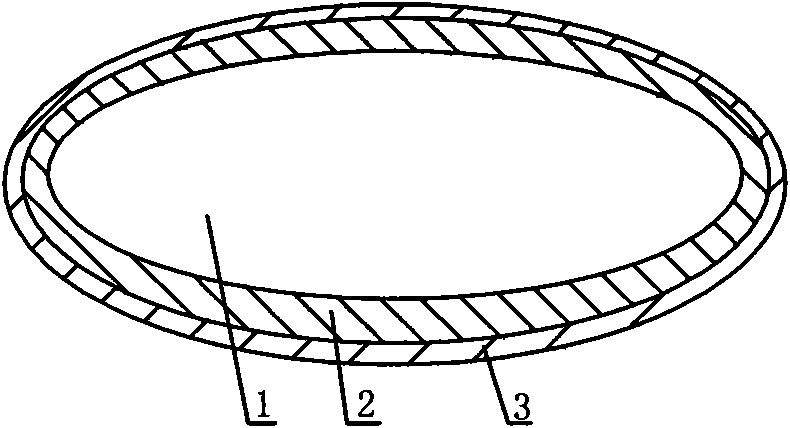
[0044] Such as figure 1 As shown, a new oral capsule preparation for the treatment of cancer and AIDS has a double-layer structure, with drug layer 1, water-soluble capsule 2 and enteric-coated capsule 3 in sequence from inside to outside. The drug layer is a mixture of protein drugs, absorption aids and protease inhibitors.
[0045] The protein drug used was interleukin-2, the absorption aids were taurodeoxycholic acid and lauroylcarnitine, and the protease inhibitor was citric acid.
[0046] The preparation method is as follows:
[0047] 1. Mix evenly 0.5 g of interleukin-2 freeze-dried product, 5 g of taurodeoxycholic acid, 5 g of lauroyl carnitine and 40 g of granulated citric acid. This amount is used to prepare 100 unit doses.
[0048] 2. The medicine prepared above is evenly wrapped in 100 water-soluble capsules according to the conventional process. Capsules can adopt any capsule material that can be dissolved in water in the prior art.
[0049] 3. Preparation of e...
Embodiment 2
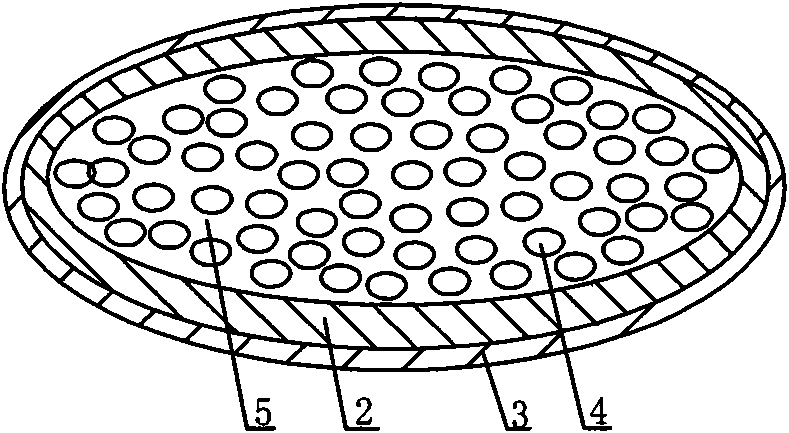
[0052] Such as figure 2 As shown, a new oral capsule preparation for the treatment of cancer and AIDS has a multi-layer structure, which consists of a drug layer 1, a water-soluble capsule 2 and an enteric-coated capsule 3 from the inside to the outside. The drug layer 1 is a mixture composed of several small capsules 4 and protease inhibitors 5, and the small capsules are coated with protein drugs and absorption aids.
[0053] The protein drug used was interleukin-2, the absorption aids were taurodeoxycholic acid and lauroylcarnitine, and the protease inhibitor was citric acid.
[0054] The preparation method is as follows:
[0055] 1. Mix 1.0 g of interleukin-2 polypeptide freeze-dried product, 2.5 g of taurodeoxycholic acid and 2.5 g of lauroyl carnitine, and make microcapsules of 100-200 microns by fluidized layer spray coating method. Figure 4 The basic process of the fluidized-bed spray coating method is shown. First use air flow 11 to make the mixture of interleuki...
Embodiment 3
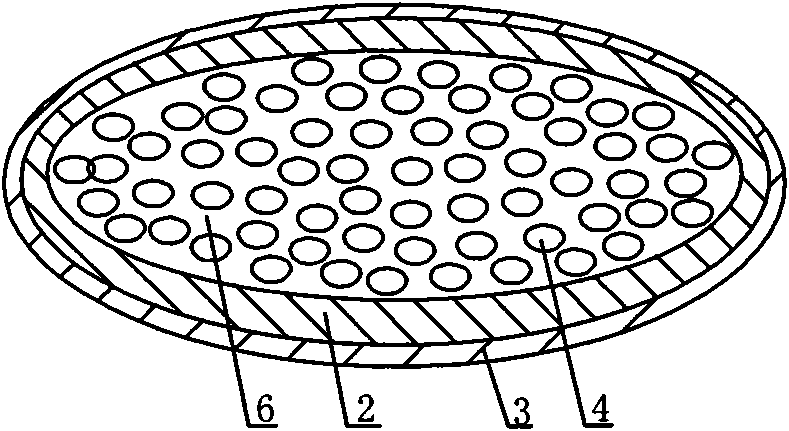
[0059] Such as image 3 As shown, a novel oral capsule preparation for treating cancer and AIDS has a multi-layer structure, including drug layer 1, water-soluble capsule 2 and enteric-coated capsule 3 from inside to outside. The drug layer 1 is a mixture composed of several small capsules 4 and a mixture 6 of protease inhibitors and some absorption aids, wherein the small capsules are coated with protein drugs and the rest of the absorption aids.
[0060] The protein drug used was interleukin-2, the absorption aids were taurodeoxycholic acid and lauroylcarnitine, and the protease inhibitor was citric acid.
[0061] The preparation method is as follows:
[0062] 1. Mix 5 grams of interleukin-2 freeze-dried product, 5 grams of taurodeoxycholic acid and 5 grams of lauroyl carnitine, and make microcapsules of 100-200 microns by fluidized layer spray coating method. Figure 4 The basic process of the fluidized-bed spray coating method is shown. First use air flow 11 to make the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com