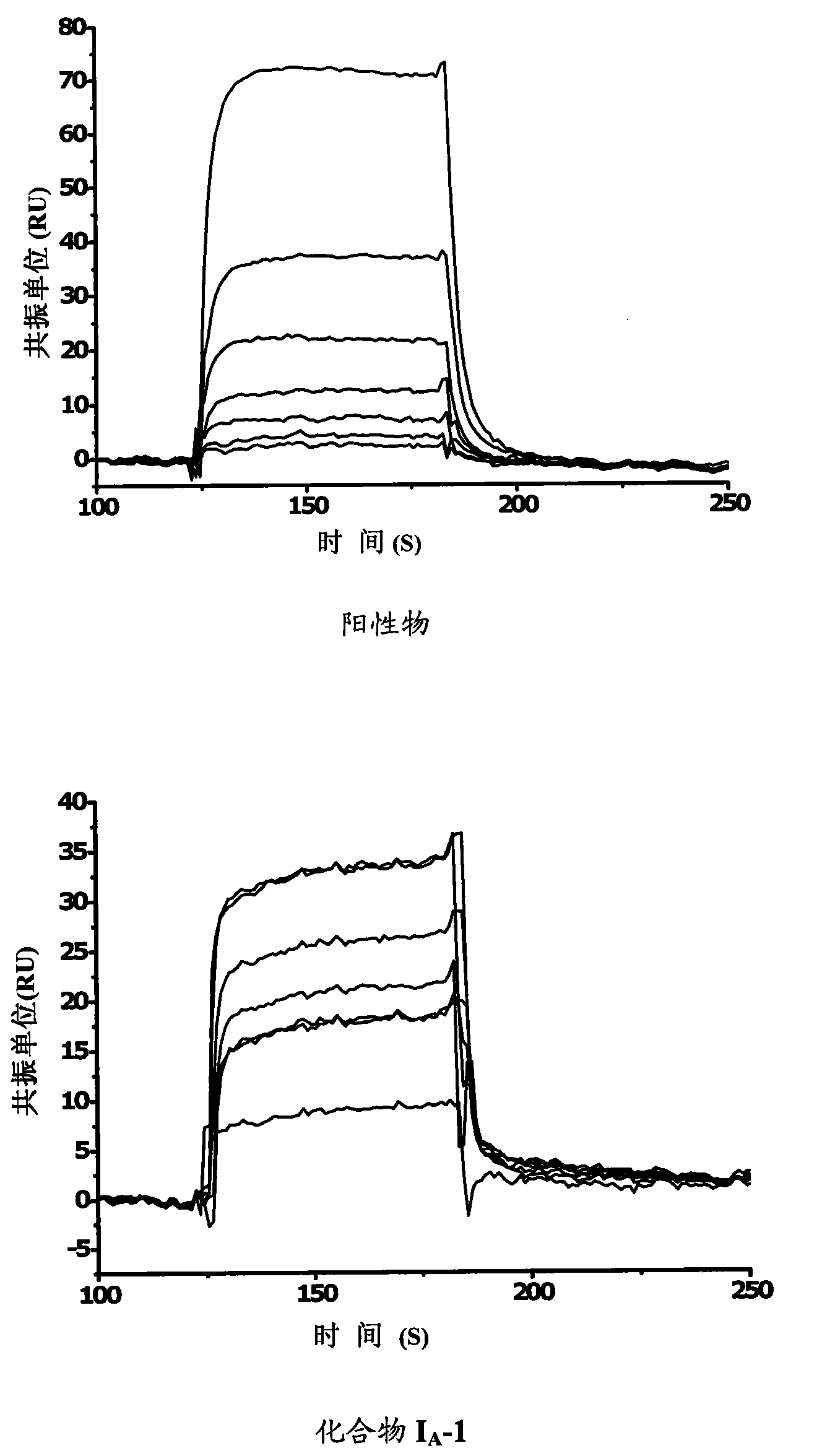
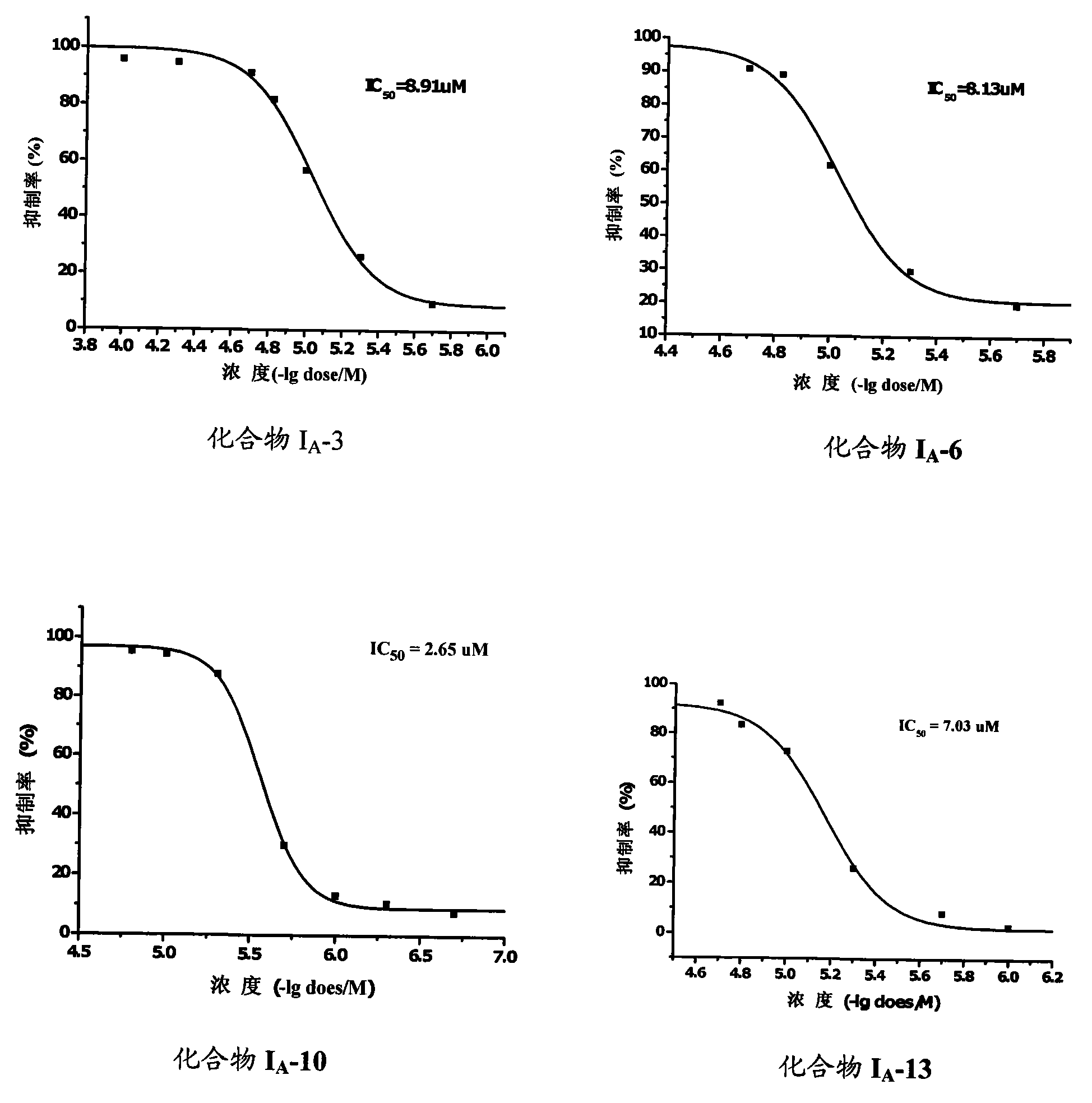
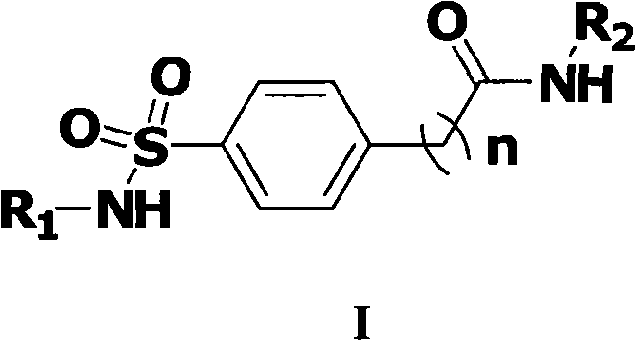
Benzene alkyl amides compound and usage thereof
A technology of benzalkonium compounds and compounds is applied in the field of benzalkonium compounds and can solve problems such as affecting the function of oocysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of methyl 3-phenylpropionate (compound II-1)
[0055] Dissolve 3.0 g of 3-phenylpropionic acid in a 50 mL round-bottomed flask filled with 30 mL of anhydrous methanol, heat to reflux at 70°C, then cool to 20°C-30°C, control this temperature, and slowly dissolve 1 mL of concentrated sulfuric acid under stirring Add it dropwise to the reaction liquid, raise the temperature to 70°C, and reflux for 5 hours. Remove the solvent by distillation under reduced pressure, add 30 mL of ethyl acetate, wash with distilled water (10 mL×3), 5% sodium bicarbonate (10 mL×3), and distilled water (10 mL×3) successively, and dry the ethyl acetate layer with anhydrous magnesium sulfate , filtered, and the solvent was removed under reduced pressure to obtain 2.9 g of methyl 3-phenylpropionate (II-1) as a colorless liquid with a yield of 88%. MS (EI) m / z 164 (M + ).
Embodiment 2
[0057] Preparation of methyl 3-(4-chlorosulfonylphenyl)propionate (compound III-1)
[0058] Add 5.5 mL of chlorosulfonic acid dropwise to 2.9 g of methyl 3-phenylpropionate (II-1), control the rate of addition, keep the temperature of the reaction solution not higher than 40°C, and continue stirring for 1.5 hours after the addition. The reaction solution was poured into 80 mL of ice water, extracted with dichloromethane (30 mL×3), washed with brine (10 mL×3) and distilled water (10 mL×3) successively, the organic phase was dried with anhydrous magnesium sulfate, filtered, and removed under reduced pressure The solvent was used to obtain 3.3 g of methyl 3-(4-chlorosulfonylphenyl)propionate (III-1) as a white solid with a yield of 72%. mp 62-64°C; MS (EI) m / z 262 (M + ).
Embodiment 3
[0060] Preparation of methyl 3-(4-(N-phenethyl-sulfamoyl)phenyl)propionate (compound IV-1)
[0061] Add 1.0 g of 2-phenylethylamine to 20 mL of pyridine, stir at 20°C-30°C, and add 1.0 g of 3-(4-chlorosulfonylphenyl)propionic acid methyl ester (III-1) in batches to In the reaction solution, after the addition, continue to stir for 2 hours, remove the solvent under reduced pressure, add an appropriate amount of water and extract with dichloromethane, wash with 6N hydrochloric acid in turn, dry the dichloromethane with anhydrous magnesium sulfate, filter, and remove the solvent under reduced pressure 0.78 g of methyl 3-(4-(N-phenethyl-sulfamoyl)phenyl)propionate (IV-1) was obtained as light yellow liquid with a yield of 60%. MS (EI) m / z 347 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com