Antilipidemic pharmaceutical compositions and process for preparation thereof
A technology of blood lipid-lowering drugs and compositions, applied in the field of oral pharmaceutical compositions, capable of solving problems such as reduced compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Particle I:
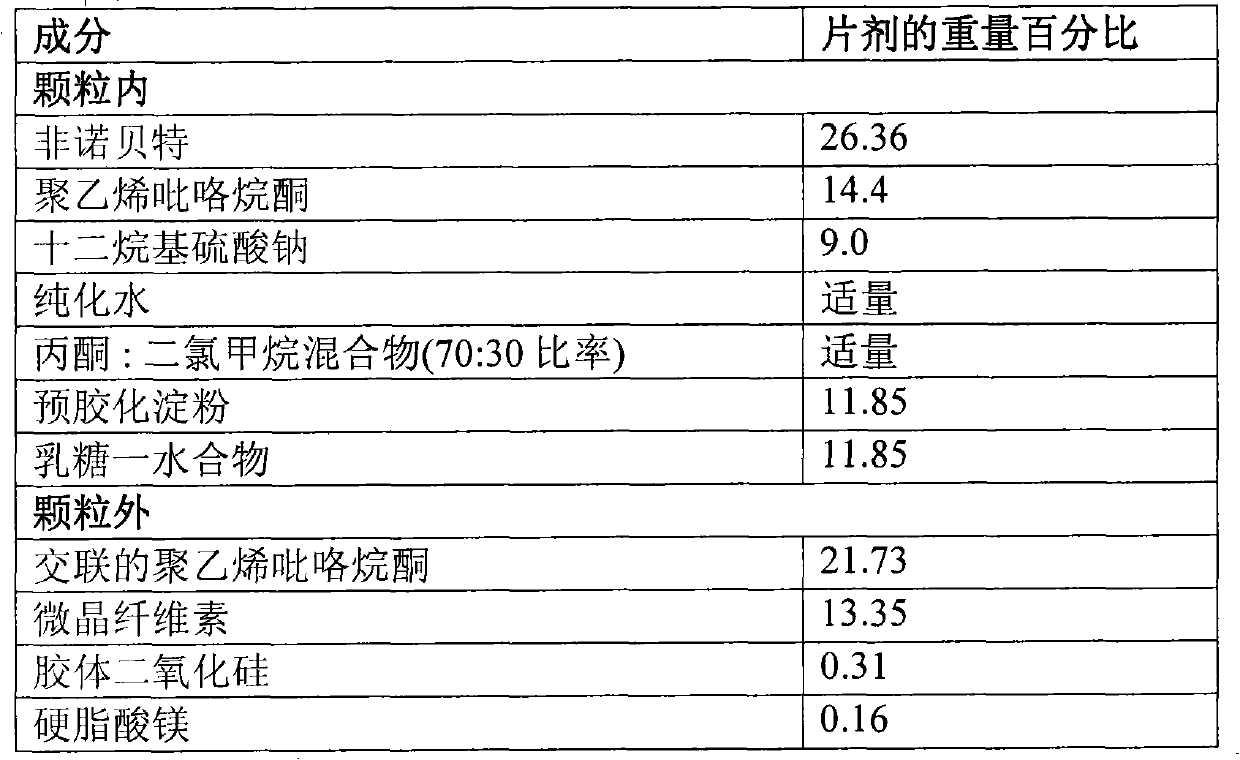
[0106] Preparation of Fenofibrate Granules I
[0107] composition
[0108]
[0109] step:
[0110] 1. Dissolve fenofibrate, polyvinylpyrrolidone and sodium lauryl sulfate in water and stir to form a clear solution.
[0111] 2. A mixture of acetone:dichloromethane (70:30 ratio) was added to the solution from step 1 while stirring and stirring was continued for 45 minutes.
[0112] 3. Spray the solution from step 2 onto the mixture of pregelatinized starch and lactose to form fenofibrate granules.
[0113] 4. The dried granules of step 3 were sieved and mixed with cross-linked polyvinylpyrrolidone, microcrystalline cellulose, colloidal silicon dioxide and magnesium stearate to form a fenofibrate granule mixture.
Embodiment 2
[0115] Particle II:
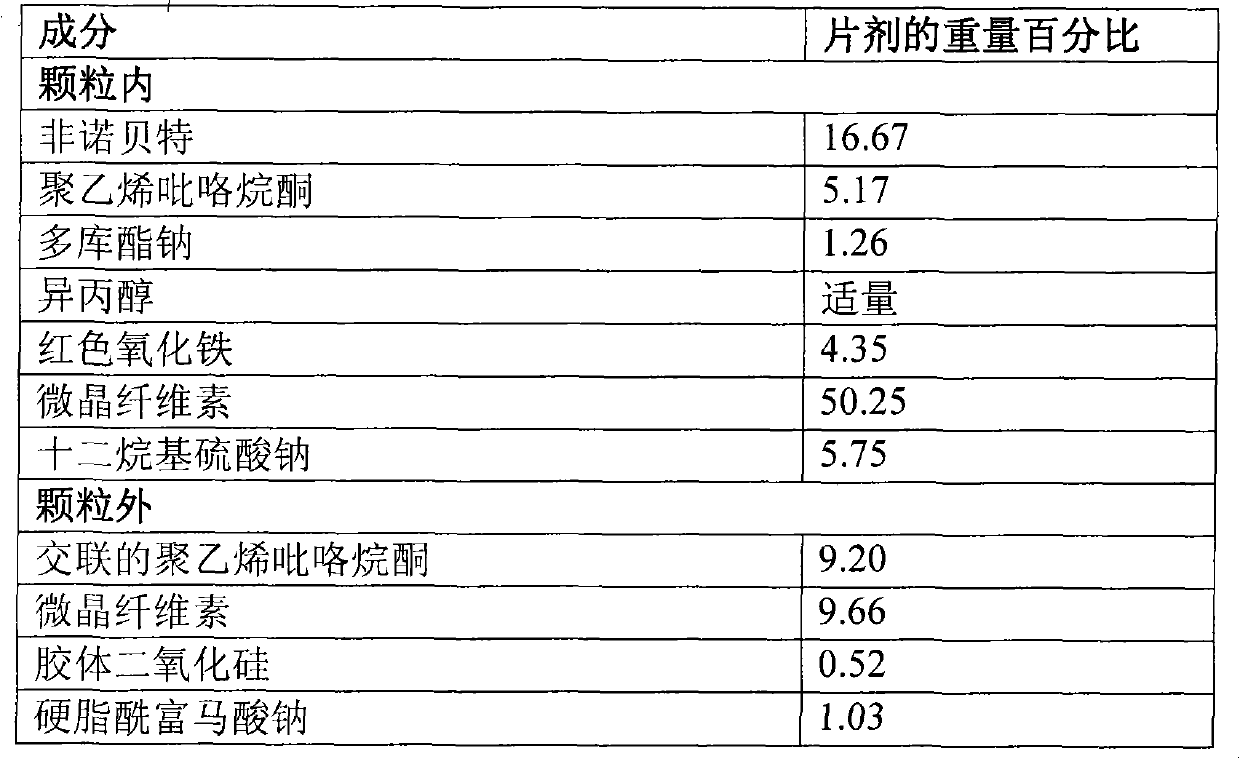
[0116] Preparation of Fenofibrate Granules II
[0117] composition
[0118]
[0119] step:
[0120] 1. Dissolve fenofibrate, polyvinylpyrrolidone, and docusate sodium in isopropanol and stir to form a clear solution.
[0121] 2. Spray the solution from step 1 onto the mixture of microcrystalline cellulose, sodium lauryl sulfate and red iron oxide to form fenofibrate granules.
[0122] 3. The dried granules from step 2 were sieved and blended with cross-linked polyvinylpyrrolidone, microcrystalline cellulose, colloidal silicon dioxide and magnesium stearate to form a fenofibrate granule mixture.
[0123] Particle III:
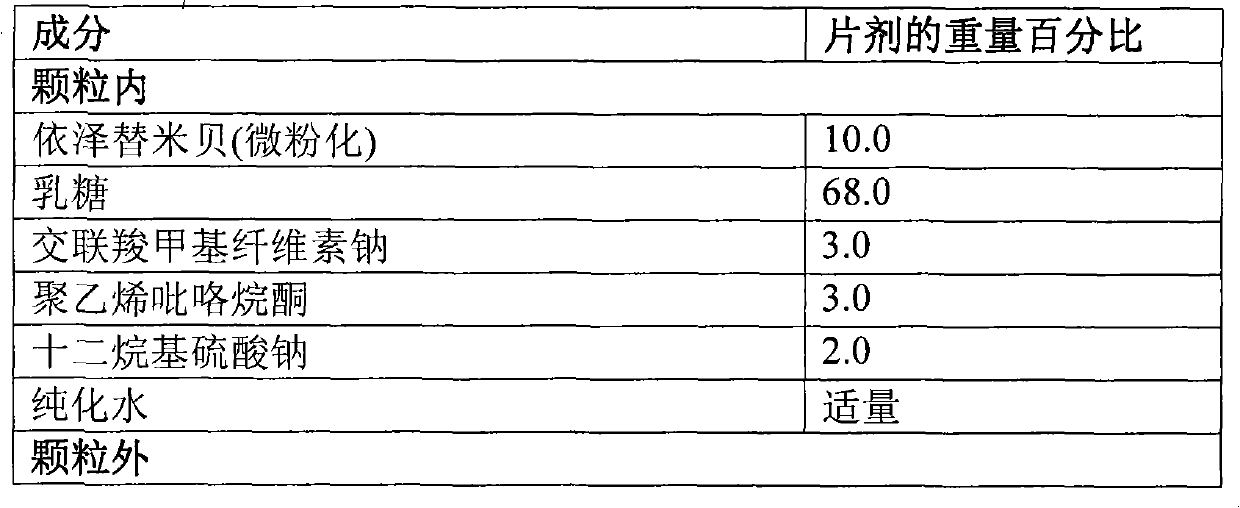
[0124] Preparation of Ezetimibe Granules
[0125] composition
[0126]
[0127]
[0128] step:
[0129] 1. Mix ezetimibe, croscarmellose sodium and lactose together to form a homogeneous mixture.
[0130] 2. Sodium lauryl sulfate and polyvinylpyrrolidone were dissolved in water and mixed with the mixture of step 1 to form e...
Embodiment 3
[0162] The fenofibrate granules of Example 1 were compressed with a special tool to form a monolithic tablet. The tablets were coated with the coating composition described above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com