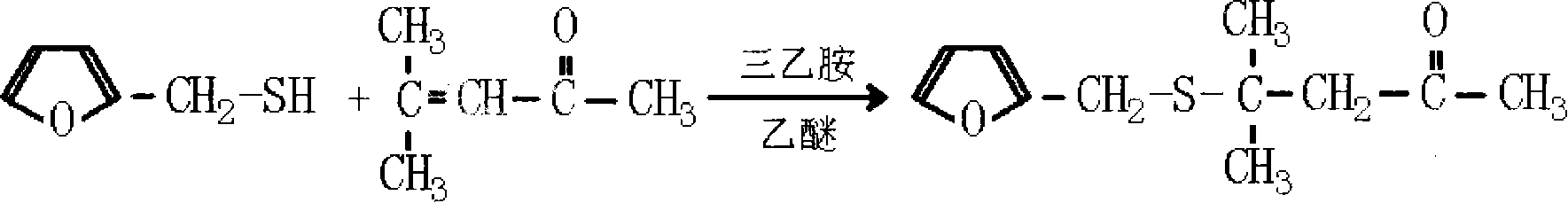
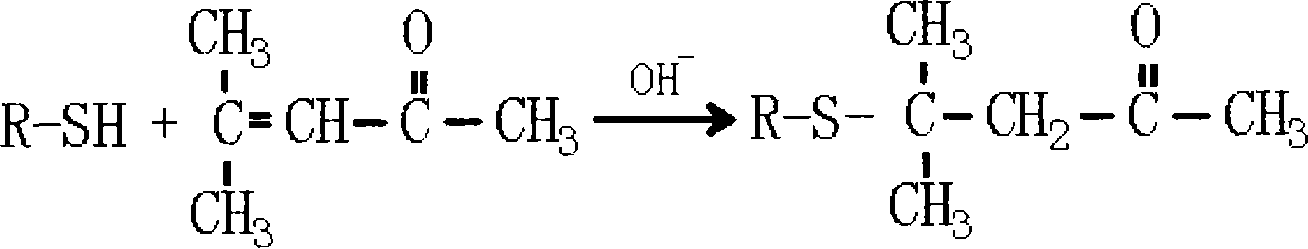Method for preparing 4-methyl-4-(2-furanmethylthio)-2-pentanone
A technology of furan methylthio and methyl groups is applied in the field of preparation of edible fragrance monomers, and can solve the problems of difficult industrialized continuous production, irritating odor of triethylamine, influence on product aroma quality, etc., so as to save the solvent recovery step, Sensory quality assurance, effect of simplified operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 9.6 grams of sodium hydroxide, 190 milliliters of water in the 500ml flask that is equipped with electric stirring, thermometer, reflux device and dropping funnel, start stirring and make sodium hydroxide dissolve, be configured into 5% sodium hydroxide aqueous solution (sodium hydroxide content is 1.2 times of theoretically calculated molar weight), then 22.8 grams of 2-furyl mercaptan (the molar ratio of 2-furyl mercaptan and mesityl oxide is 1: 1.1) was added dropwise at 10 ℃, and then at the same temperature Add 21.6 grams of mesityl oxide (1.1 times the theoretically calculated molar weight), react the feed solution at 50°C for 3.5 hours, cool the feedstock to 15°C, adjust the pH to 6 with 10% sulfuric acid, separate the organic phase, and wash twice , and the separated organic phase was dried again, and the fraction at 86°C / 40Pa was collected by distillation to obtain the product 4-methyl-4-(2-furylmethylthio)-2-pentanone with a yield of 81%.
Embodiment 2
[0037] Add 12.8 grams of sodium hydroxide, 115 milliliters of water in the 500ml flask that is equipped with electric stirring, thermometer, reflux device and dropping funnel, start stirring and make sodium hydroxide dissolve, be configured into 10% sodium hydroxide aqueous solution (sodium hydroxide The content is 1.6 times of the theoretically calculated molar weight), then 22.8 grams of 2-furyl mercaptan (the molar ratio of 2-furyl mercaptan and mesityl oxide is 1: 1.5) was added dropwise at 20 ° C, and then at the same temperature Add 29.4 grams of mesityl oxide (1.5 times the theoretically calculated molar weight), react the feed solution at 70°C for 1.5 hours, cool the feedstock to 10°C, adjust the pH to 6.5 with 5% sulfuric acid, separate the organic phase, and wash twice , and the separated organic phase was dried again, and the fraction at 86°C / 40Pa was collected by distillation to obtain the product 4-methyl-4-(2-furylmethylthio)-2-pentanone with a yield of 80.56%.
Embodiment 3
[0039] Add 15.7 grams of potassium hydroxide, 142 milliliters of water in the 500ml flask that is equipped with electric stirring, thermometer, reflux device and dropping funnel, start stirring and make potassium hydroxide dissolve, be configured into 10% potassium hydroxide aqueous solution (potassium hydroxide The content is 1.4 times of the theoretically calculated molar weight), then 22.8 grams of 2-furyl mercaptan (the molar ratio of 2-furyl mercaptan and mesityl oxide is 1: 1.3) was added dropwise at 15 ° C, and then at the same temperature Add 25.5 grams of mesityl oxide (1.3 times the theoretically calculated molar weight), react the feed solution at 60°C for 2 hours, cool the feedstock to 10°C, adjust the pH to 7 with 8% sulfuric acid, separate the organic phase, and wash twice , and the separated organic phase was dried again, and the fraction at 86° C. / 40 Pa was collected by distillation to obtain the product 4-methyl-4-(2-furylmethylthio)-2-pentanone with a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com