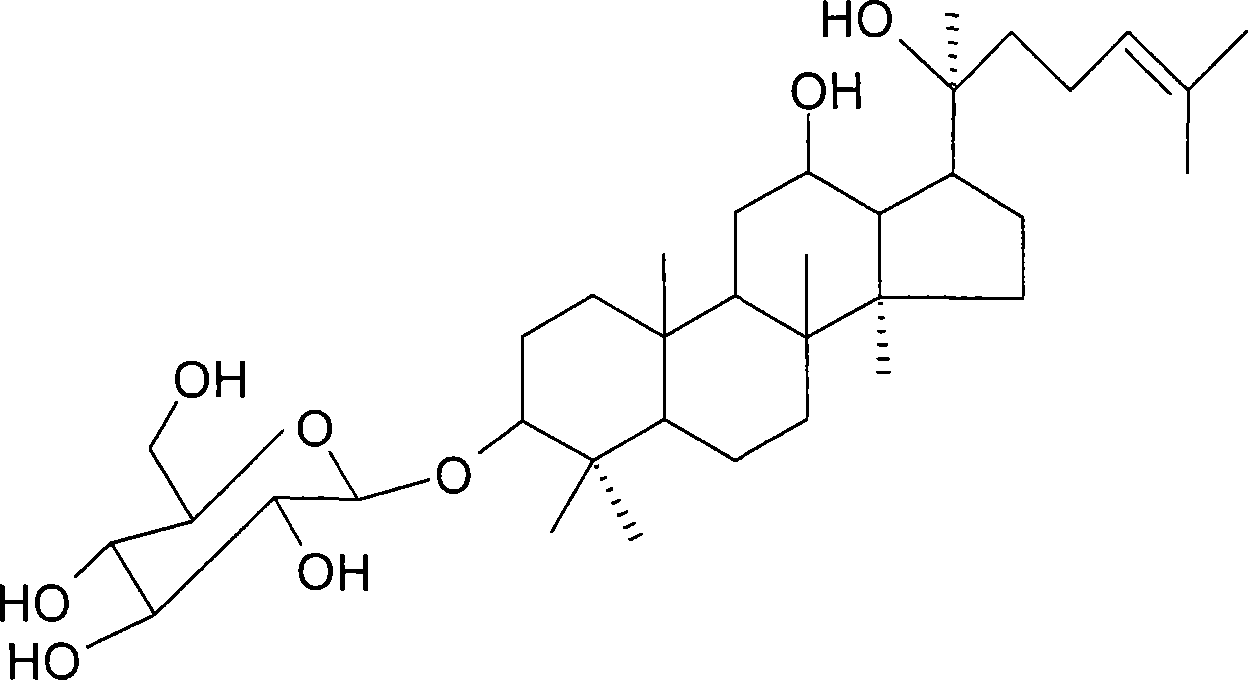
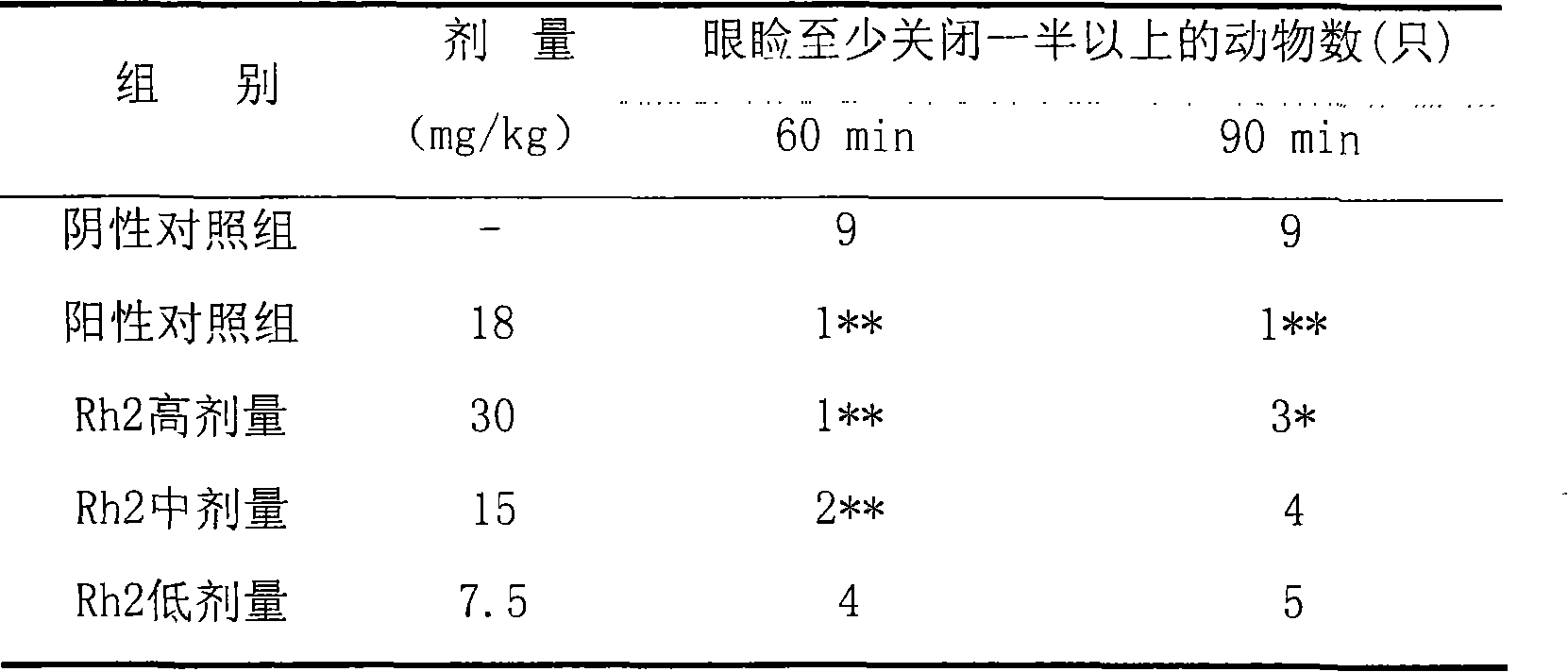
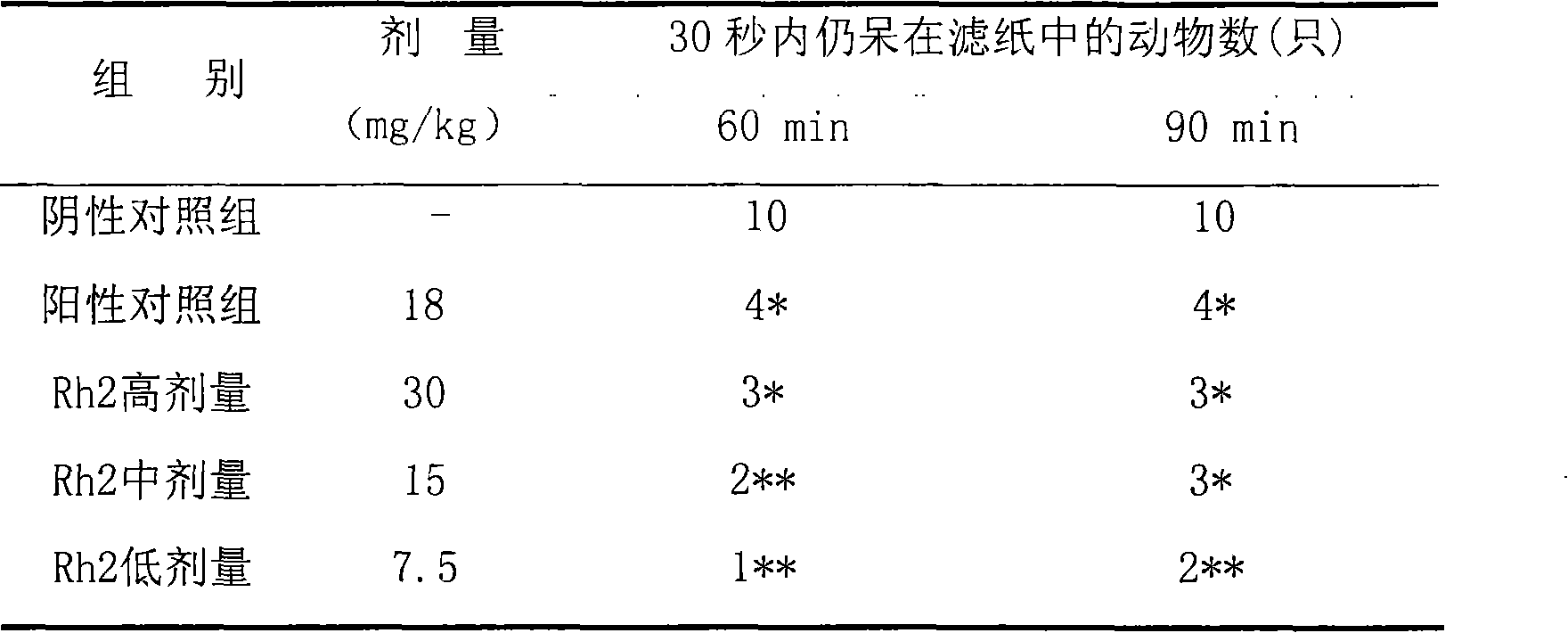
Application of 20(S)-ginsenoside Rh2 compound in preparing antidepressant medicament
A ginsenoside and antidepressant technology, which can be applied in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve the problems of unreported antidepressant effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Preparation of 20(S)-ginsenoside Rh2 ordinary capsules
[0129] Prescription: 20(S)-Ginsenoside Rh2 25mg
[0130] Lactose 170mg
[0131] Carboxymethyl Starch Sodium 5mg
[0132] 5% povidone (K30) in 70% ethanol solution
[0133] Take 20(S)-ginsenoside Rh2, lactose, and sodium carboxymethyl starch through a 80-mesh sieve and mix evenly, add 5% povidone K30 in 70% ethanol solution to make a soft material, pass through a 40-mesh sieve to granulate. 50 ~ 60 ℃ oven blast drying. The dry granules are sieved through a 40-mesh sieve. Fill No. 1 capsules according to the prescribed amount, each containing 20(S)-ginsenoside Rh2 25mg.
Embodiment 2
[0135] Preparation of 20(S)-ginsenoside Rh2 ordinary capsules
[0136] Prescription: 20(S)-Ginsenoside Rh2 25mg
[0137] Microcrystalline Cellulose 170mg
[0138] Crospovidone 5mg
[0139] 5% povidone (K30) in 70% ethanol solution
[0140] Take 20(S)-ginsenoside Rh2, microcrystalline cellulose, and cross-linked povidone through a 80-mesh sieve and mix evenly, add an appropriate amount of 5% povidone K30 in 70% ethanol solution to make a soft material, pass through a 40-mesh sieve to granulate . 50 ~ 60 ℃ oven blast drying. The dry granules are sieved through a 40-mesh sieve. Fill No. 1 capsules according to the prescribed amount, each containing 20(S)-ginsenoside Rh2 25mg.
Embodiment 3
[0142] Preparation of 20(S)-ginsenoside Rh2 ordinary capsules
[0143] Prescription: 20(S)-Ginsenoside Rh2 25mg
[0144] Povidone (K30) 175mg
[0145] Proper amount of ethanol
[0147] Take 20(S)-ginsenoside Rh2, povidone, add appropriate amount of ethanol and heat to dissolve, put the rotary evaporator to remove ethanol, cool, pulverize, pass through a 40-mesh sieve, add the prescribed amount of magnesium stearate, and mix well. Fill No. 1 capsules according to the prescribed amount, each containing 20(S)-ginsenoside Rh2 25mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com