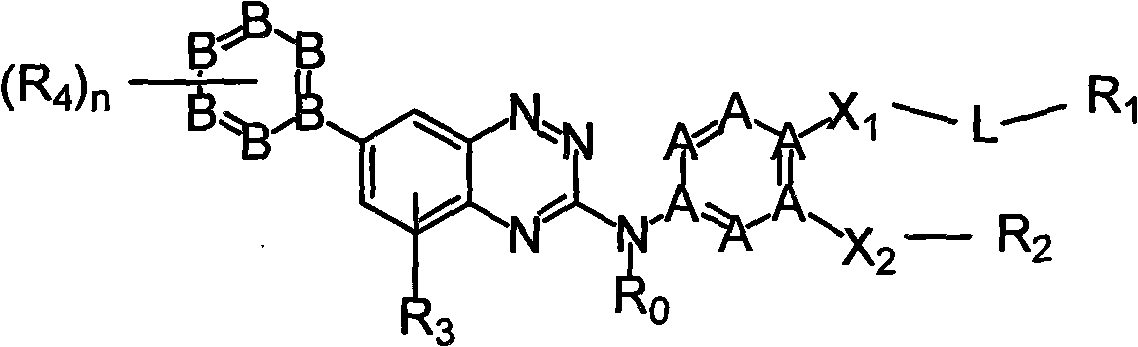
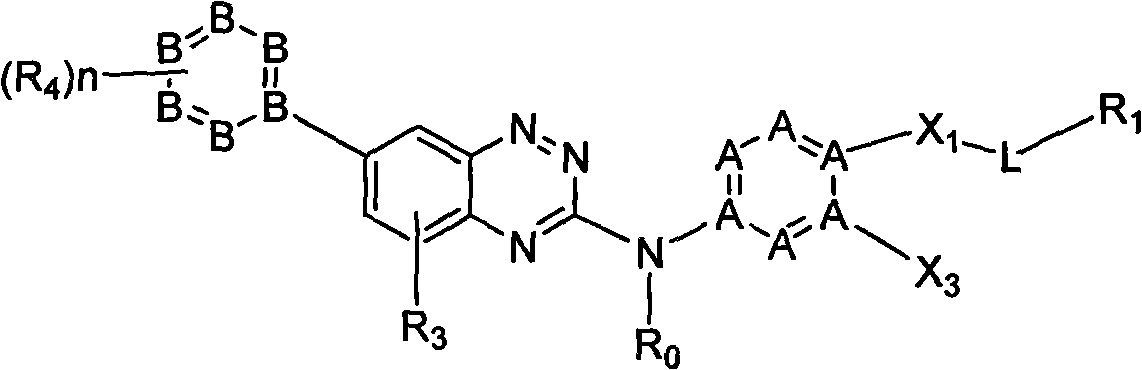
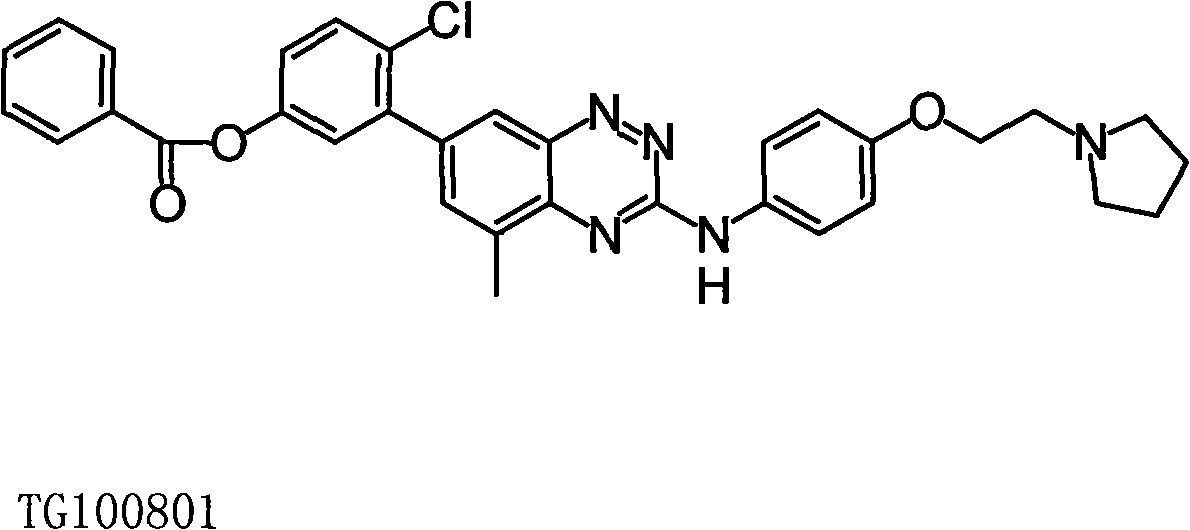
Micromolecule phentriazine dioxin-like compound and medicine by taking compound as active ingredient
A benzotriazine and compound technology, applied in the field of small-molecule benzotriazine compounds and medicines using the compound as an active ingredient, can solve problems such as high price and retinal detachment, and achieve the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of Compound I
[0046] 1. Weigh 10.5g (66.2mmol) of 2-methyl-5-nitro-aniline and place it in a single-necked bottle, add 150ml of CCl4, stir to dissolve, then add 15.6g (831.5mmo) of NBS under ice bath, After the dropwise addition was completed, the ice bath was removed, heated to reflux for 3.5 hours, cooled to room temperature instantly, filtered, and the filter cake was washed three times with 12ml of water and dried to obtain 24.3g of a yellow solid with a yield of 92%.
[0047] 2. Take by weighing 10.2g (41.2mmol) of the compound of the previous step reaction, dissolve it in 20ml of glacial acetic acid, heat to reflux, then add dropwise the hydrochloric acid solution of 6.8g (320mmol) of aminoacetonitrile in the reaction solution, and the dropwise addition is completed Afterwards, the temperature was maintained at 120 degrees for overnight reaction. The reaction solution was cooled, and the pH was adjusted to 12 with a strong base, then the mixture was r...
Embodiment 2
[0055] Synthesis of Compound II
[0056] 1. Weigh 10g (65mmol) of 2-methyl-5-nitro-aniline and place it in a single-necked bottle, add 150ml of CCl4, stir to dissolve, then add 14.6g (81.5mmo) of NBS under ice bath, drop After completion, the ice bath was removed, heated to reflux for 3.5 hours, cooled to room temperature instantly, filtered, and the filter cake was washed three times with 10 ml of water and dried to obtain 23.2 g of a yellow solid with a yield of 90%.
[0057] 2. Take by weighing 9.2g (40mmol) of the compound of the previous step reaction, dissolve it in 20ml of glacial acetic acid, heat to reflux, then add dropwise the hydrochloric acid solution of 6.8g (320mmol) of aminoacetonitrile in the reaction solution, after the dropwise addition , the temperature was maintained at 120 degrees overnight. The reaction solution was cooled, and the pH was adjusted to 12 with a strong base, then the mixture was refluxed for 2 hours, filtered, and the cake was passed thro...
Embodiment 3
[0065] Synthesis of compound III
[0066] Take 5.08g of compound-2, dissolve it in 20ml of THF, add 1ml of triethylamine, stir to dissolve, add dropwise the HTH solution of benzoyl chloride to the reaction solution. The reaction was carried out at room temperature for 3.5 hours, followed by TLC until the reaction was complete. Add 15ml of water to the reaction solution, extract with ethyl acetate, wash the organic phase 3 times with saturated sodium bicarbonate (3*20ml), wash with saturated brine, and dry over anhydrous sodium sulfate. Purification on a silica gel column yielded 7.09 g of a yellow solid. The yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com