Method for synthesizing key intermediate of rosuvastain calcium
A technology for rosuvastatin calcium and intermediates, which is applied in the field of synthesizing rosuvastatin calcium key intermediates, can solve the problems of purification, storage, inconvenience in use, low production efficiency, and high operating costs, and achieves advantages that are beneficial to subsequent reactions, Improve product quality and avoid the effect of rectification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
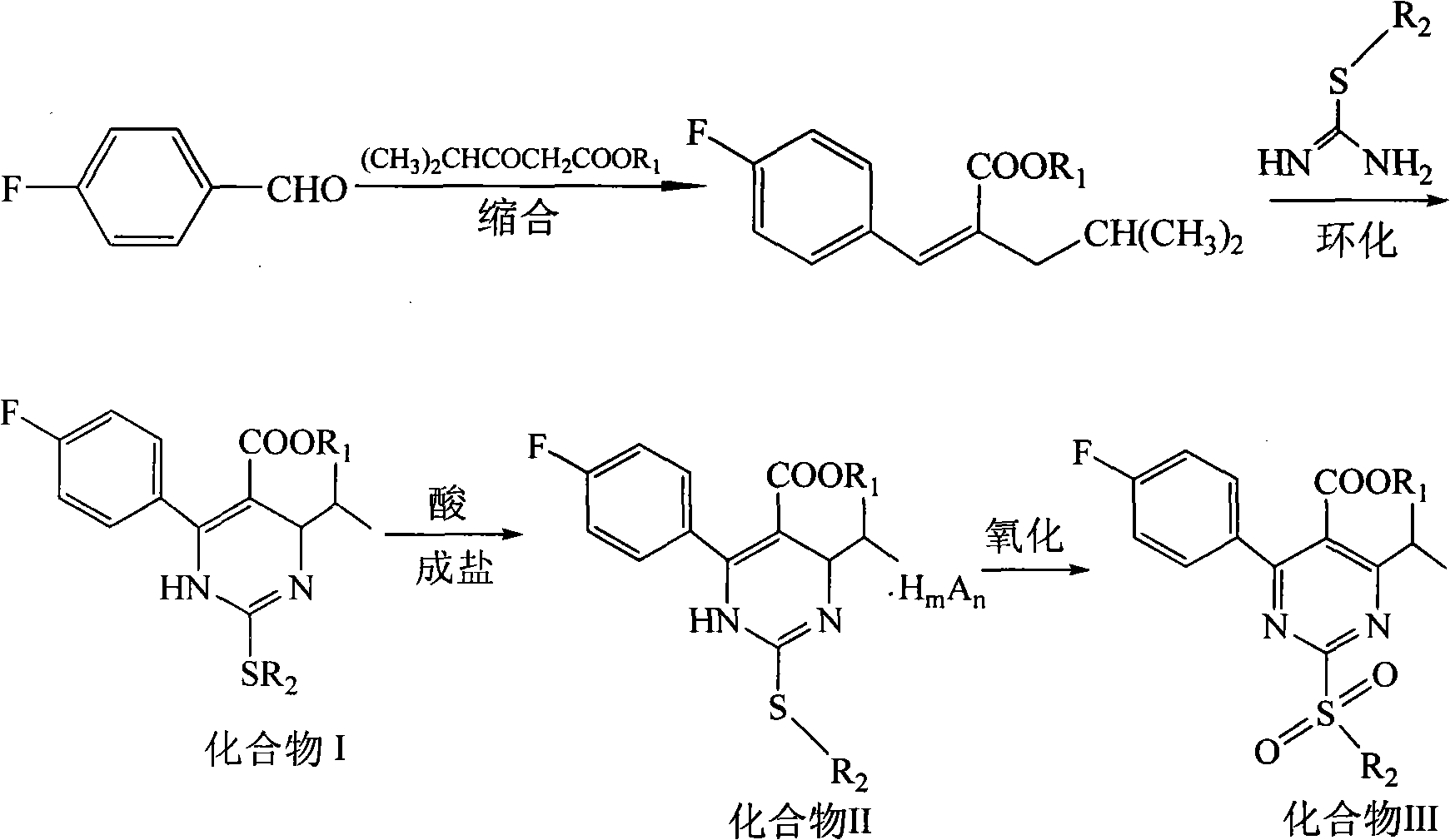
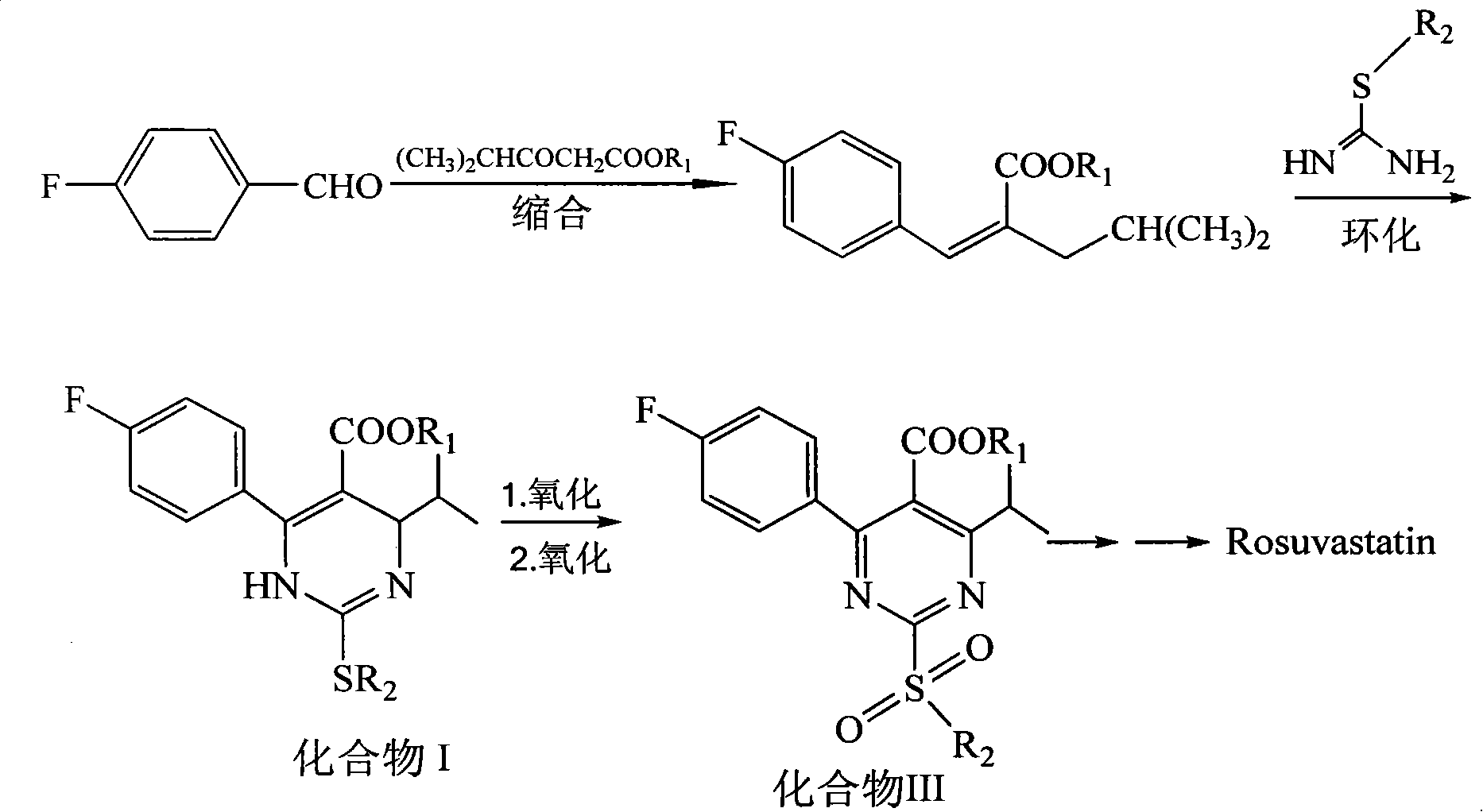
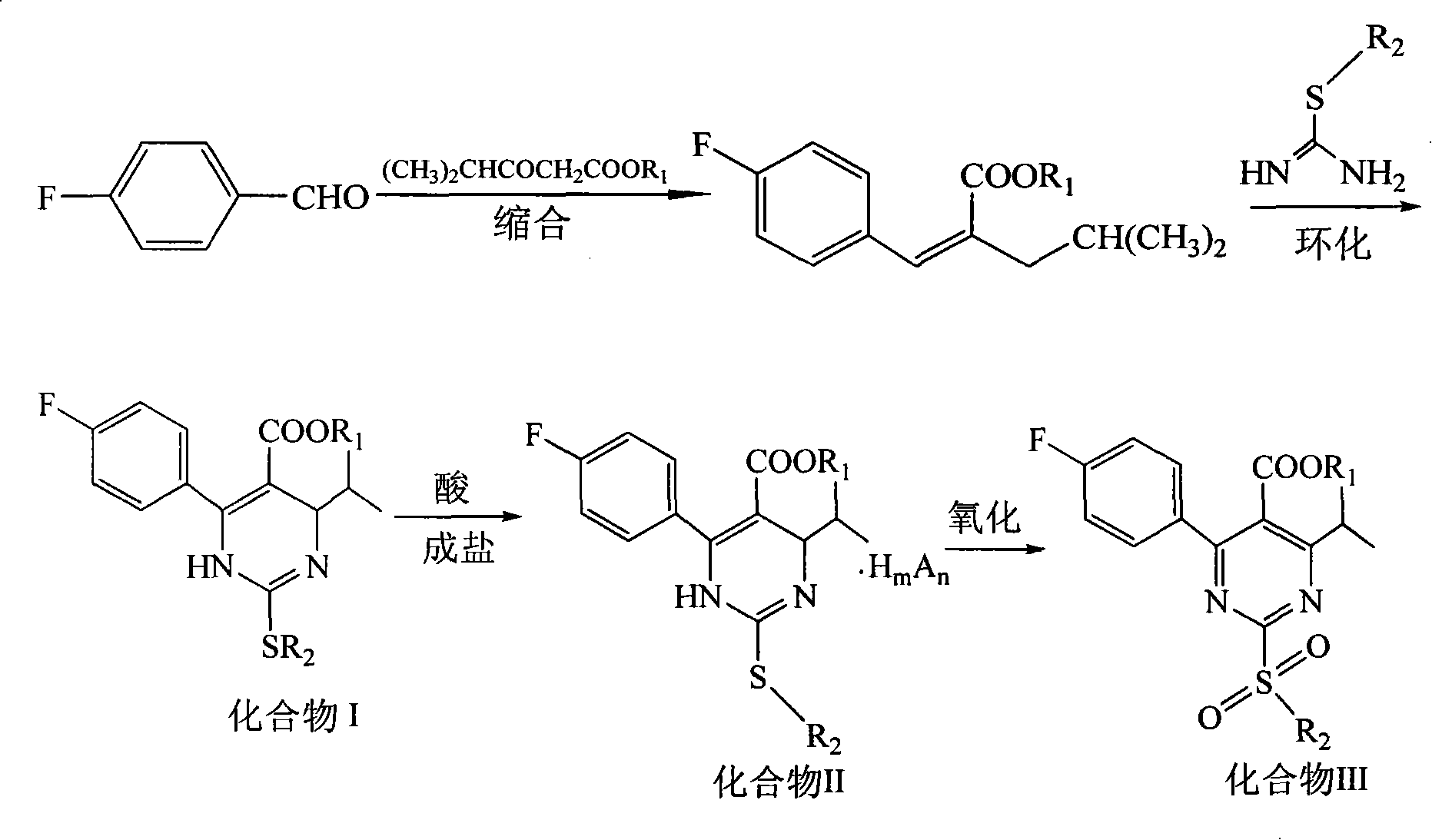
[0031] Synthesis of 3-(4-fluorophenyl)-2-isobutyryl methyl acrylate: in a reaction flask, add p-fluorobenzaldehyde (18.4g, 0.15mol), methyl isobutyryl acetate (21.6g, 0.15 mol), acetic acid 2ml, piperidine 2ml, toluene 50ml, heated to reflux for 15h. Cool down to room temperature, add 20 ml of 10% sodium carbonate aqueous solution, stir, and separate the liquids. The organic phase was washed with 20 mL of 10% sodium carbonate aqueous solution, the aqueous phases were combined, the organic phase was extracted with toluene, the obtained organic layer was washed with water, washed with saturated brine, and separated. After concentration, 35.0 g of a brownish-red oil was obtained, which was 3-(4-fluorophenyl)-2-isobutyryl methyl acrylate, which could be put into the next reaction without rectification.
[0032] Compound II (R 1 =R 2 =Me, the salt-forming agent is hydrochloric acid) synthetic: add the compound 3-(4-fluorophenyl)-2-isobutyryl methyl acrylate (35.0g, 0.148mol) tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com