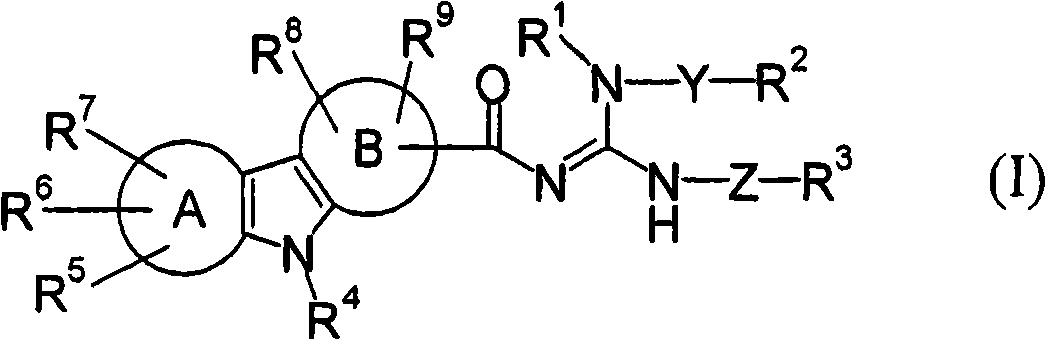
Acylguanidine derivative
An alkyl and phenyl technology, applied in the field of substituted guanidine derivatives, can solve the problems of undisclosed dementia, schizophrenia, cognitive impairment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0193] (Preparation of raw material compound)
[0194] The raw material compounds (1) to (4) in the above production methods can be produced, for example, by the following methods, known methods or modified methods thereof.
[0195] (Raw material synthesis 1)
[0196] [chemical 15]
[0197]
[0198] (In the formula, Q and U represent leaving groups respectively, and any one represents -Br, -Cl, -I or -O-SO 2 -CF 3 etc., the other side means -B(OH) 2 or B(O-lower alkyl) 2 Wait. R 10 Represents a protecting group for a carboxyl group such as a lower alkyl group or benzyl group. )
[0199] In the raw material compound (1), R 4 The compound that is H can be directly prepared by the above-mentioned reaction route, or by the -OR of the thus prepared compound (1a) 10 Prepared by converting to a leaving group.
[0200] Among them, the coupling reaction can be passed through "Synthetic Communications", (UK), 1981, Volume 11, p.513-519; "Synlett", (Germany), 2000, Volume 6...
Embodiment
[0220] Hereinafter, a method for producing a compound contained in formula (I), which is an active ingredient of the present invention, will be described as an example. In addition, the production method of the compound used as a raw material is demonstrated as a production example. The production method of compound (I) is not limited to the production methods shown in the specific examples below, and can also be produced by a combination of these production methods, known production methods or their improved methods.
[0221] In the description of the following preparation examples and the tables described later, the following abbreviations are used for the measured values by mass spectrometry.
[0222] ESI+:ESI-MS[M+H] + ;ESI-:ESI-MS[M-H] - ;FAB+: FAB-MS[M+H] + or FAB-MS[M] + ;FAB-:FAB-MS[M-H] - ;APCI+:APCI-MS[M+H] + ;APCI-:APCI-MS[M-H] - ;EI+:EI[M] + .
preparation example 1
[0224] By reacting methyl 3-nitro-4-{[(trifluoromethyl)sulfonyl]oxy}benzoate with phenylboronic acid, potassium phosphate, tetrakis(triphenylphosphine)palladium in DMF with heating , to obtain methyl 2-nitrobiphenyl-4-carboxylate. FAB+: 258.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com