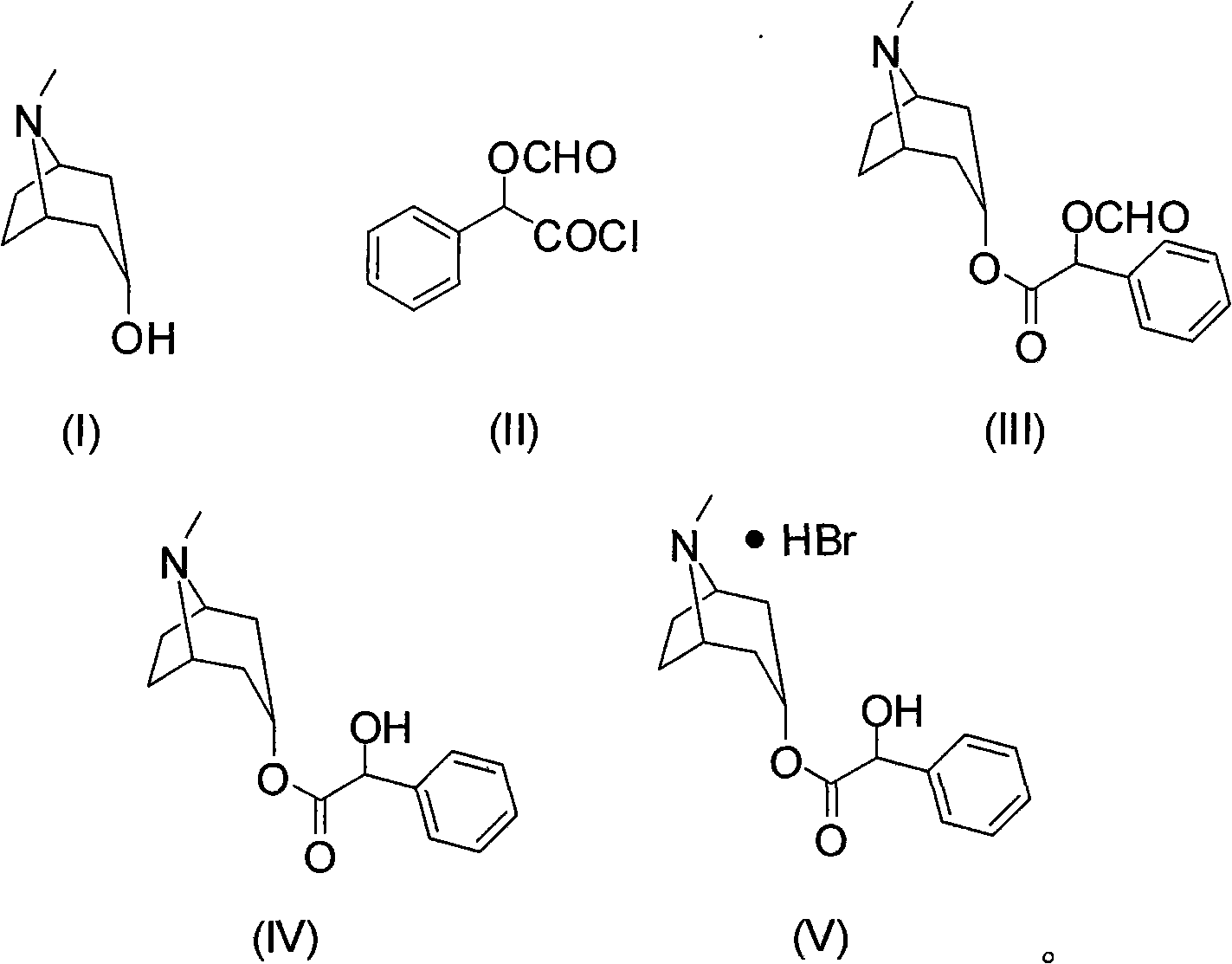
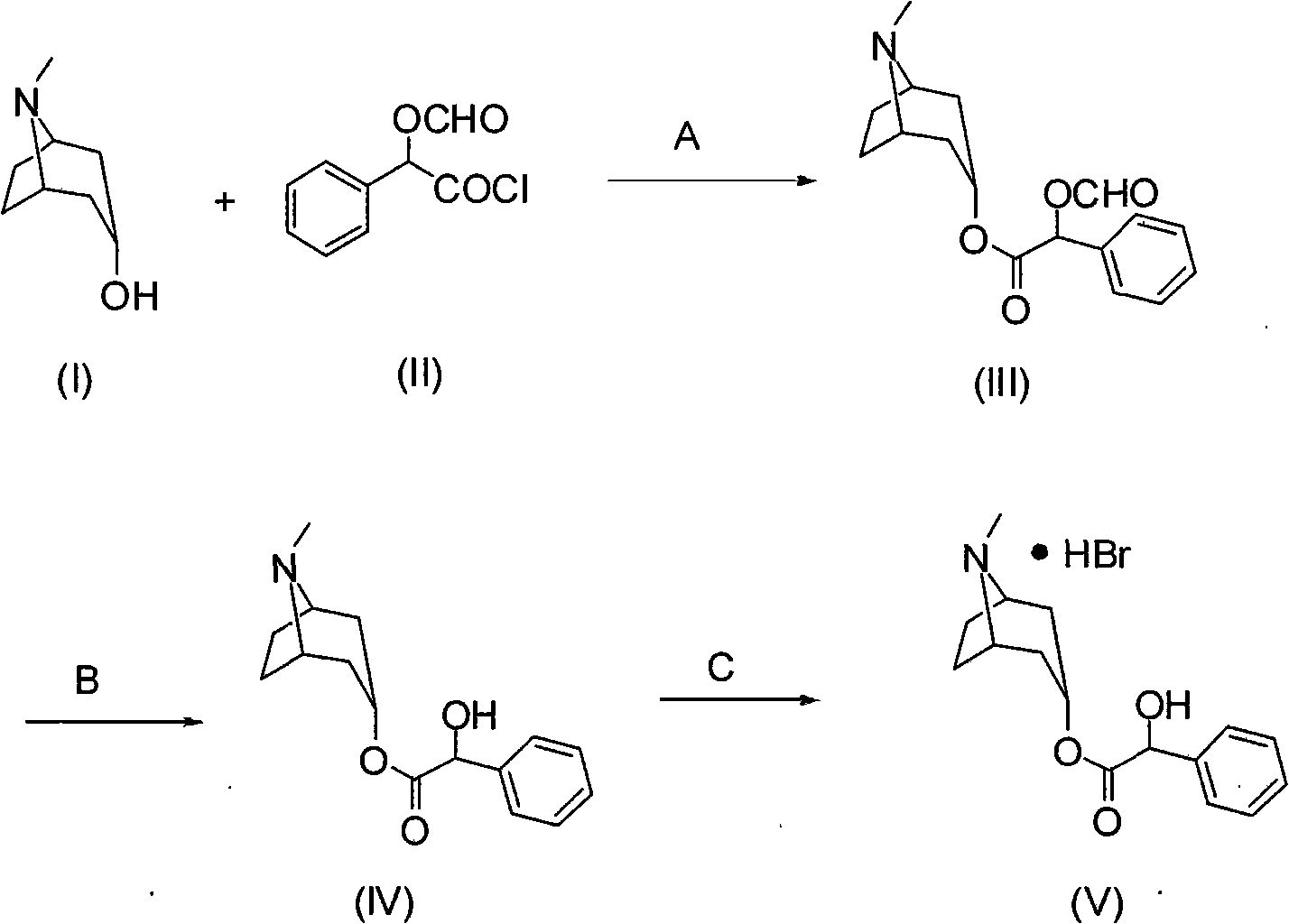
Synthesis method of homatropine hydrobromide
A kind of hydrobromide homatropine and synthetic method technology, applied in the direction of organic chemistry, etc., can solve problems such as difficult large-scale industrial production, unstable quality of finished products, mild reaction conditions, etc., to avoid the use of toxic solvent benzene, The effect of low production cost and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of compound (III)
[0024] Add 10g of tropinol and 80mL of dichloromethane into a 250mL three-necked flask equipped with mechanical stirring, reflux condenser and thermometer, stir to dissolve, add 17g of O-formylmandeloyl chloride dropwise, and stir at room temperature for 5 hours. After the reaction, the solvent was distilled off under reduced pressure to obtain 20.5 g of crude compound (III).
Embodiment 2
[0025] Embodiment 2: the preparation of compound (III)
[0026] Add 10g of tropinol, 14g of triethylamine, and 80mL of dichloromethane into a 250mL three-necked flask equipped with a mechanical stirrer, a reflux condenser, and a thermometer, stir to dissolve, add 17g of O-formylmandeloyl chloride dropwise, and stir at room temperature for 5 hours Rear. After the reaction was completed, it was washed with water, and the organic layer was separated and concentrated to obtain 20.6 g of crude compound (III).
Embodiment 3
[0027] Embodiment 3: the preparation of compound (III)
[0028] Add 10g of tropinol, 14g of triethylamine, and 80mL of toluene into a 250mL three-necked flask equipped with a mechanical stirrer, a reflux condenser and a thermometer, stir to dissolve, add 17g of O-formylmandelic acid chloride dropwise, and stir for 8 hours at room temperature. After the reaction was completed, it was washed with water, and the organic layer was separated and concentrated to obtain 21.0 g of crude compound (III).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com