Method for producing epothilone derivatives by means of selective catalytic epoxidation
A technology for epothilone and derivatives, applied in the field of novel selective epoxidation, can solve problems such as poor selectivity, unsatisfactory epothilone derivatives, and extensive attack on external double bonds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
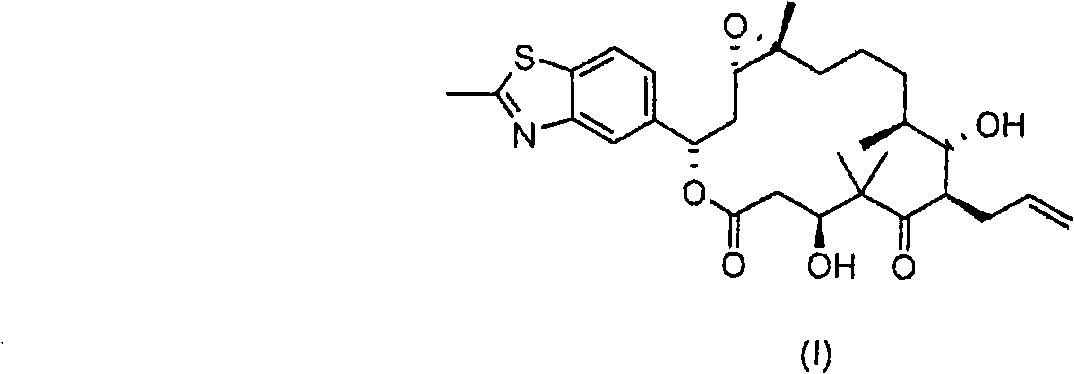
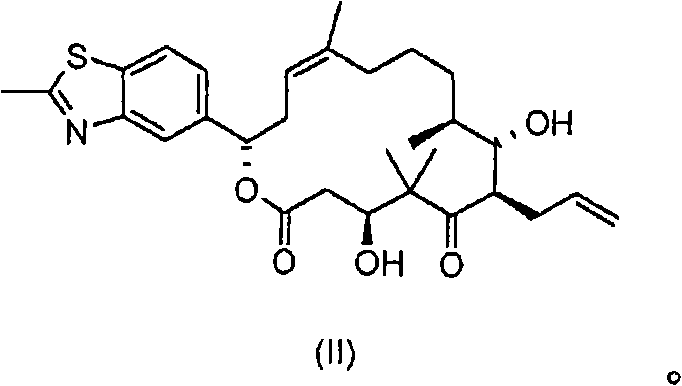
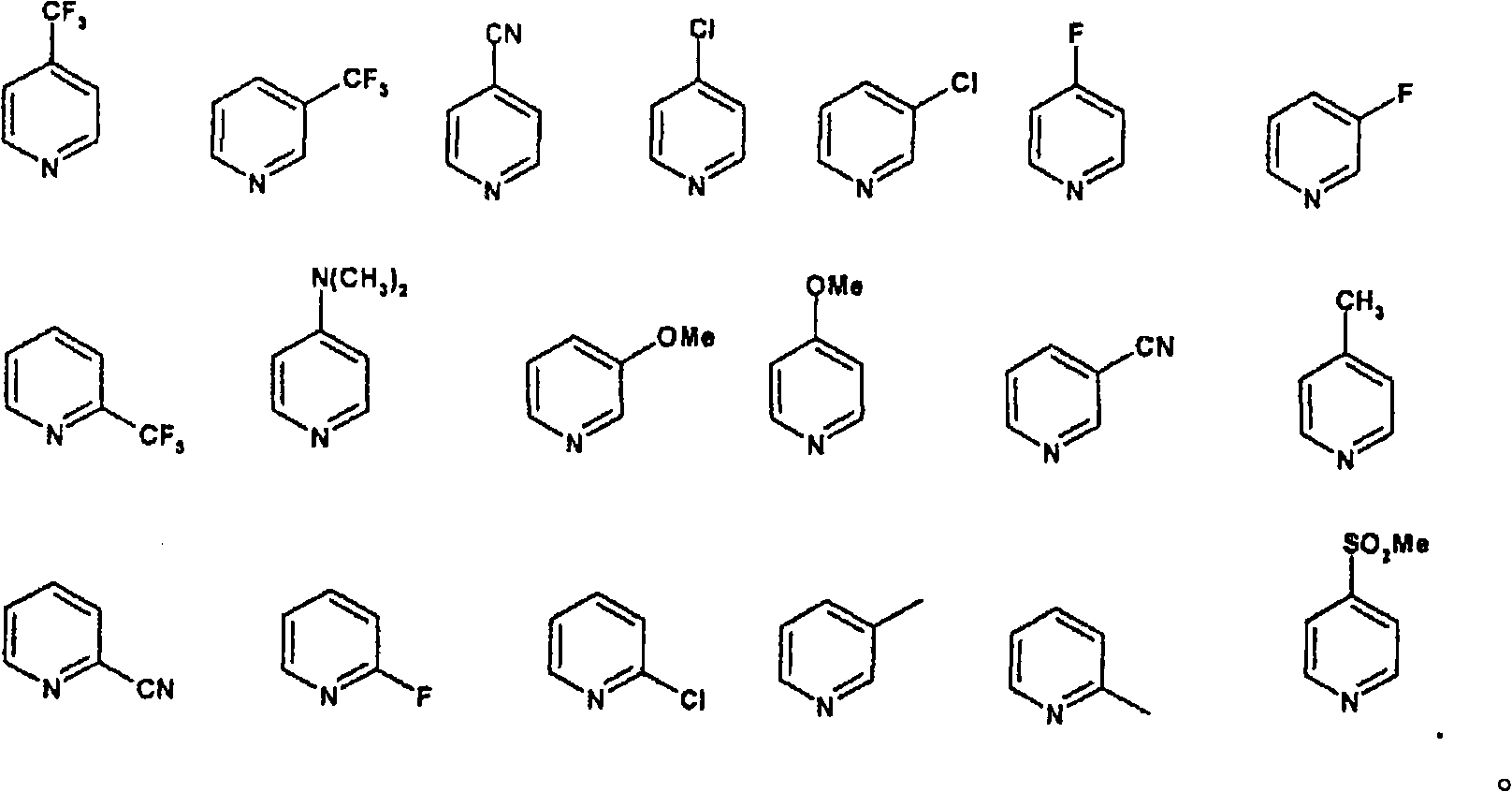
[0153] 1.000 kg of diene of formula II (prepared according to WO 00 / 66589), 14.17 g (3 mol%) of rhenium trioxide and 35.5 g (18 mol.%) of 4-cyanopyridine were dissolved in 10 liters of dichloromethane , and then cooled to -50 °C. 579 ml of a 30% aqueous hydrogen peroxide solution (3 eq.) was added, and the mixture was stirred at -50°C for about 70 hours. The reaction was followed to completion by HPLC. As soon as the precursor substance (compound of formula II) falls below 1%, the reaction is stopped by adding 580 ml of a 20% strength aqueous solution of sodium thiosulfate. Then a further 7000 ml of sodium thiosulfate solution are added and then warmed to +10°C. The mixture is stirred at +10° C. for 1 hour, the organic phase is separated off and the aqueous phase is re-extracted with 5000 ml of dichloromethane. The combined organic phases were washed with 5000 ml of saturated aqueous sodium chloride solution. The organic phase was concentrated in vacuo. The residue was fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com