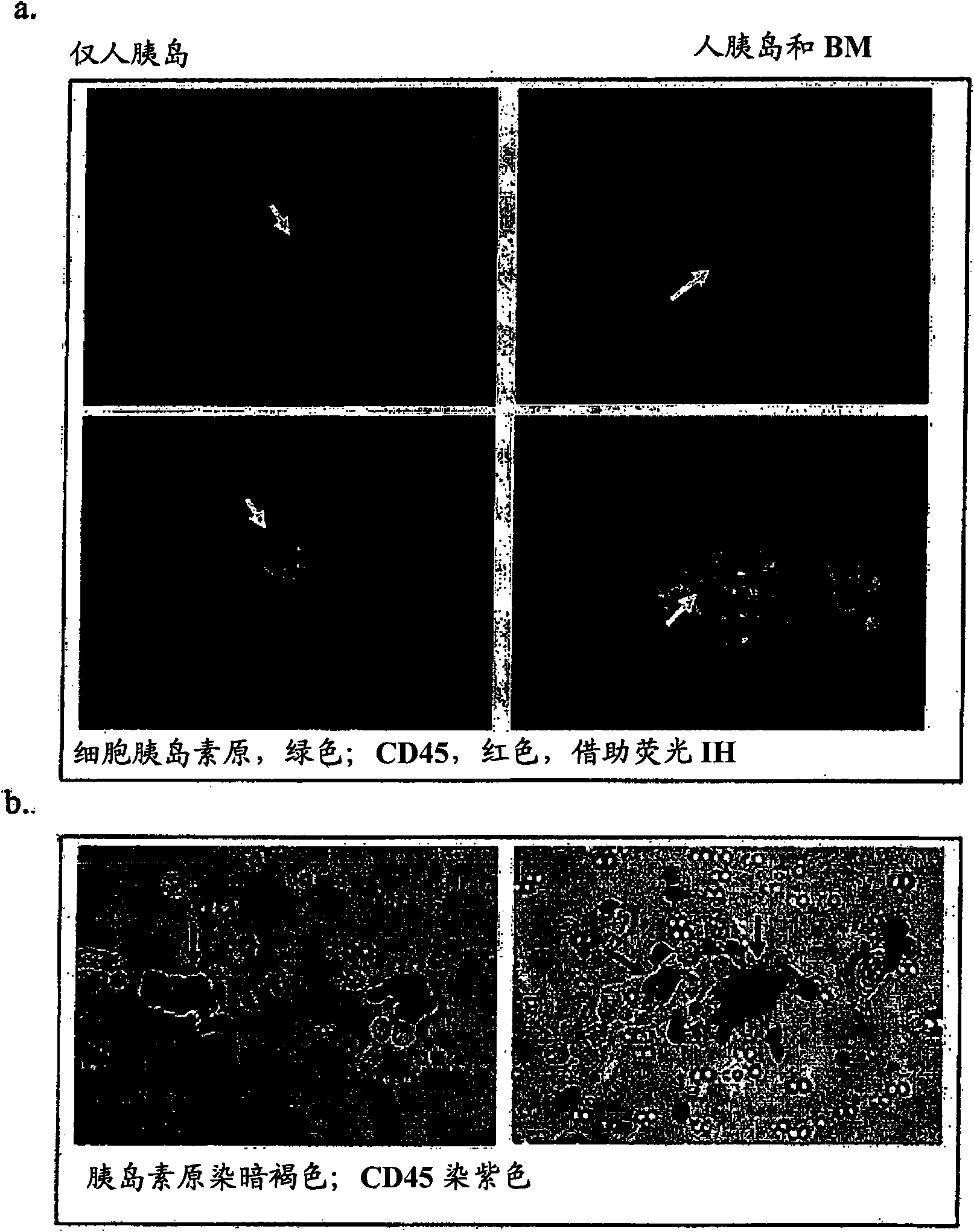
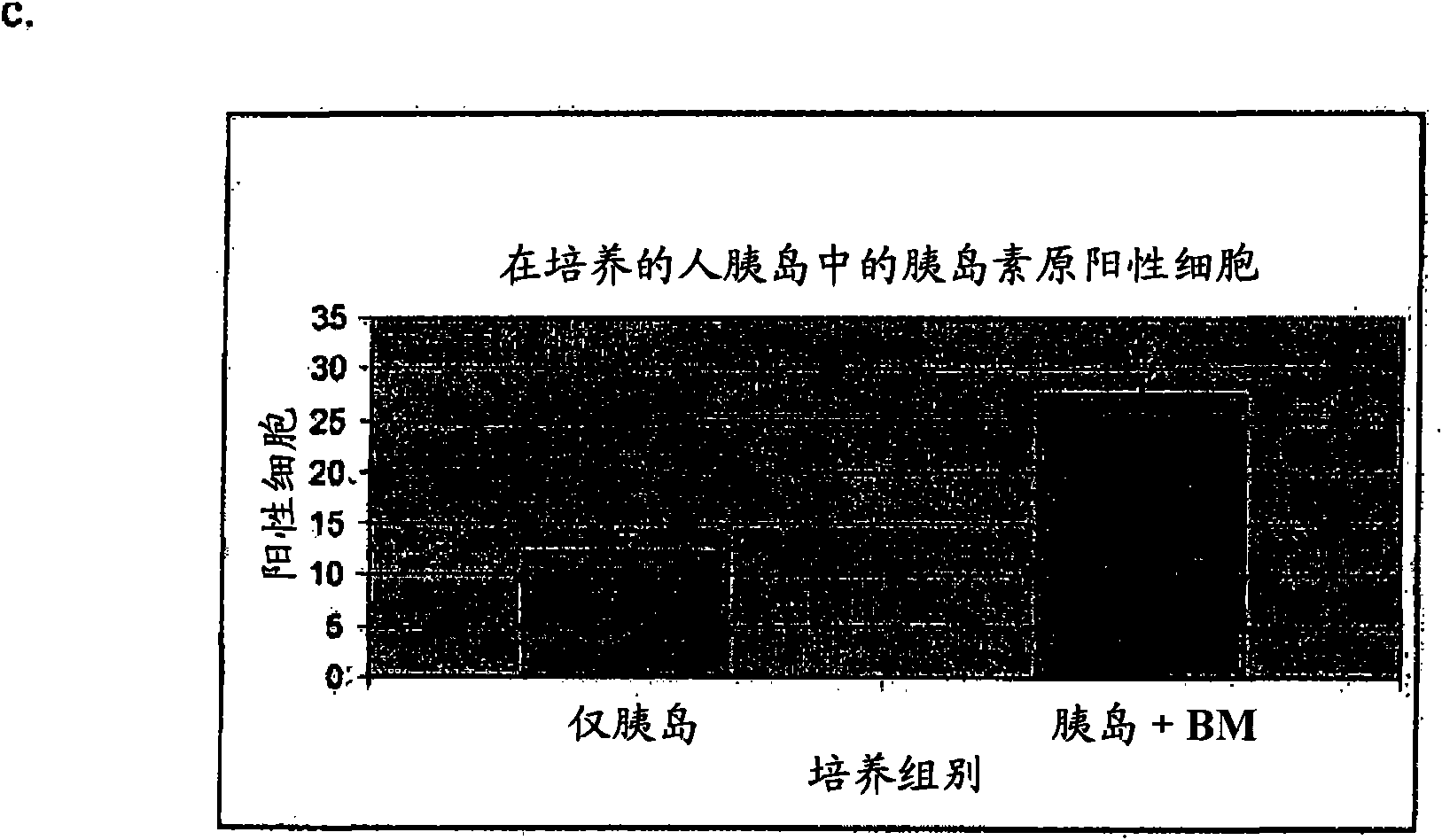
Use of bone marrow cells for long term culture of pancreatic islet cells
A technology of bone marrow cells and islet cells, applied in bone/connective tissue cells, pancreatic cells, tissue culture, etc., can solve problems such as difficulty in maintaining viable islets and expanding islet tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 - cell staining method
[0056] Methods of staining cells and immunofluorescence using fluorescent and chemical dyes are well known to those skilled in the art. Numerous examples include such dyeing methods. Exemplary cell staining methods are provided. Cells grown on chamber slides were fixed with 3% paraformaldehyde and then exposed to 10% normal goat serum. Slides were blotted without washing, primary antibody mix was applied, and slides were incubated overnight at 4°C in a humidified chamber. Slides were washed 3 times before exposure to secondary antibody for 45 minutes at room temperature. After washing, diluted secondary antibody was applied and slides were incubated for 15 minutes. The slides are then washed thoroughly with PBS, and the above process is optionally repeated with a second fluorescent color (eg, DAPI) and / or a third antigen detection antibody. At the end of the process, cover the slides with fluorescent mounting medium and a coversli...
Embodiment 2
[0057] Example 2 - Co-cultivation of human bone marrow and human islet β cells
[0058] Human islet tissue from normal donors was obtained from the Islet Resource Centers of the ICR Basic Science Islet Distribution Program (ICR Basic Science Islet Distribution Program, Human Islet Laboratory, University of Pennsylvania (Philadelphia, PA)). (ICR)), Joslin Diabetes Center (Boston, MA) and City of Hope National Medical Center (Duarte, CA). Use of these cells was approved by the IRB and ICR committees of Roger Williams Hospital.
[0059] Human bone marrow from normal donors was obtained after signing an appropriate informed consent form that had been approved by the Roger Williams Hospital Institutional Review Committee (IRB). Pass through Ficoll-Paque according to the manufacturer's directions TM Plus (Amersham Biosciences; Amersham, UK) isolated bone marrow mononuclear cells. Cells were then washed twice with 5% FCS / PBS and resuspended in medium (see below). Cell viability w...
Embodiment 3
[0069] Example 3 - Evaluation of islet function in an insulin release assay
[0070] Islet cell function was assessed by measuring insulin release with and without glucose challenge. Media was collected twice a week and stored at -80°C until basal insulin release was measured by ELISA.
[0071] The high glucose challenge assay was performed weekly as follows: media was collected and cultured cells were washed once with RPMI media. The medium was then replaced with high glucose (20 mM) RPMI 1640 for 15 and 30 minutes. Media was finally collected and stored at -80°C until insulin assay by ELISA.
[0072] Insulin concentrations in specimens (cell culture medium or tissue extracts) were detected using a human insulin ELISA kit (Linco Research, St. Charles, MO) according to the manufacturer's instructions. Briefly, insulin standards and appropriately diluted (1:50-1:500) samples were added to insulin antibody-coated 96-well microtiter plates and incubated at 4°C for 2 hours. Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com