Technology of preparing diethyl carbonate by urea alcoholysis method
A technology of diethyl carbonate and urea alcohol, which is applied in the field of urea alcoholysis to synthesize diethyl carbonate, can solve the problems of short life and low catalyst activity, and achieve the effects of reduced operating costs, high catalytic activity, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 (adopts thermal decomposition method to prepare lead-potassium composite metal oxide catalyst)
[0018] Weigh lead carbonate 6.70g and potassium nitrate 5.15g, make the mass ratio of lead oxide and potassium oxide in the catalyst be 7: 3, adopt mechanical grinding method to mix it evenly, and roast at 900 ℃ in the muffle furnace for 6 Hours, lead-potassium composite metal oxide catalyst was prepared.
[0019] Catalyst catalyzed urea alcoholysis synthesis DEC reaction with above-mentioned prepared:
[0020] (1) Add 200mL (3.425mol) absolute ethanol in the 500mL high-pressure reactor that is equipped with distillation column, 30g (0.337mol) ethyl carbamate, the lead-potassium composite metal oxide prepared above 1.6g (accounting for total reaction system The mass percentage is 0.84%);
[0021] (2) Feed nitrogen into the reactor to replace the air therein. Then, pressurize with nitrogen, and use a back pressure valve to control the pressure during the react...
Embodiment 2-6
[0025] Embodiment 2-6 (preparation of binary composite metal oxide catalyst by thermal decomposition method)
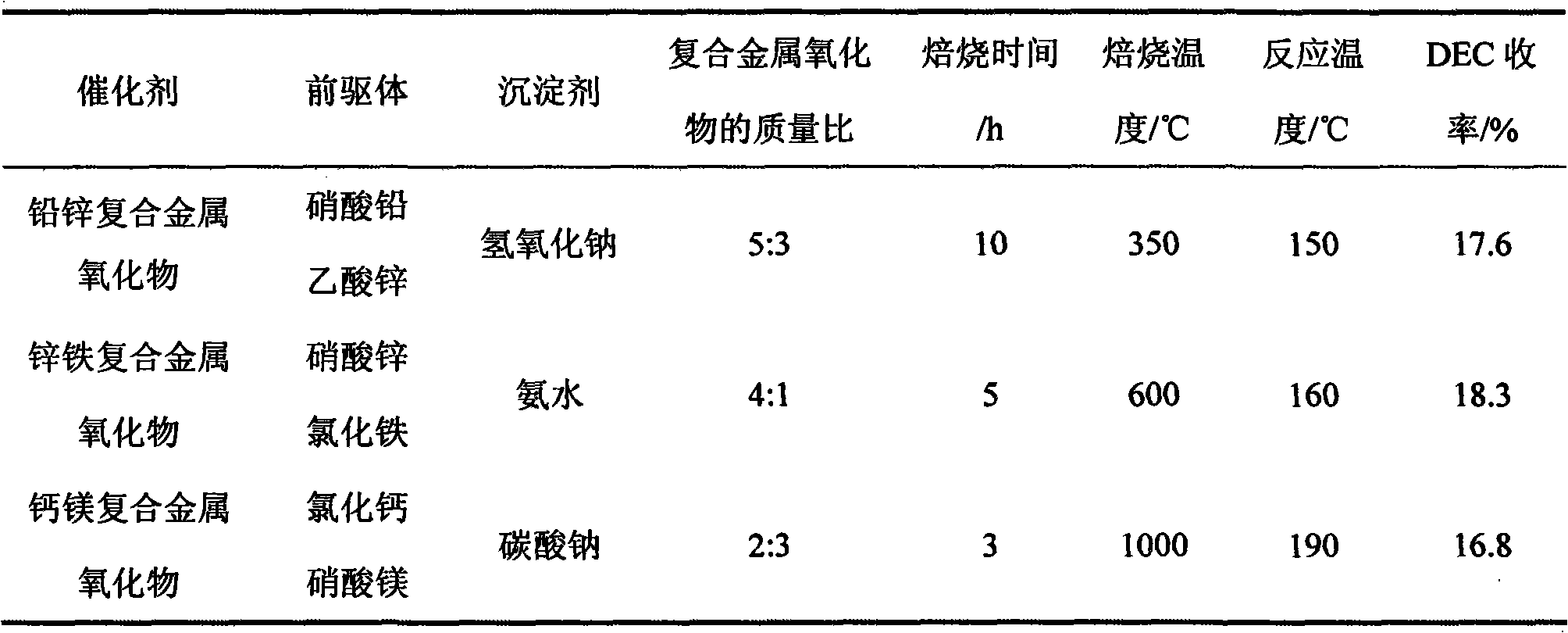
[0026] The preparation conditions of the selected precursors and catalysts are shown in Table 1. The catalysts prepared above were used to catalyze the synthesis of DEC by urea alcoholysis. Except for the catalyst and its dosage, the other reaction conditions were the same as in Example 1.
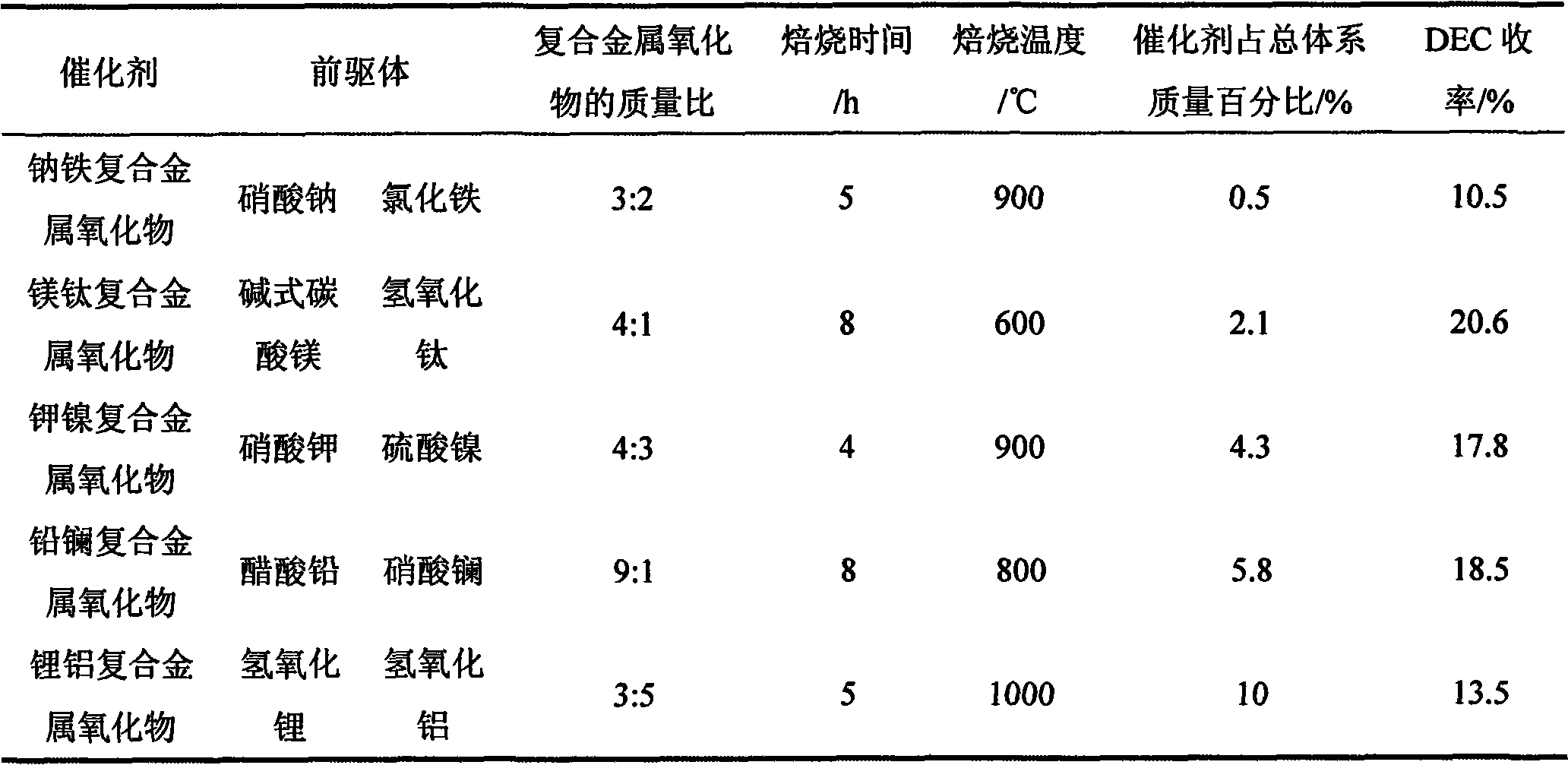
[0027] Table 1 The preparation conditions of different composite metal oxide catalysts and the influence of the mass percentage of the catalyst in the total system on the performance of the catalyst
[0028]
Embodiment 7-10
[0029] Embodiment 7-10 (preparation of binary composite metal oxide catalyst by thermal decomposition method)
[0030] The preparation conditions of the selected precursors and catalysts are shown in Table 2. The catalysts prepared above were used to catalyze the synthesis of DEC by the urea alcoholysis method. Except for the catalyst and the ratio of raw materials, the other reaction conditions were the same as in Example 1. Table 2.
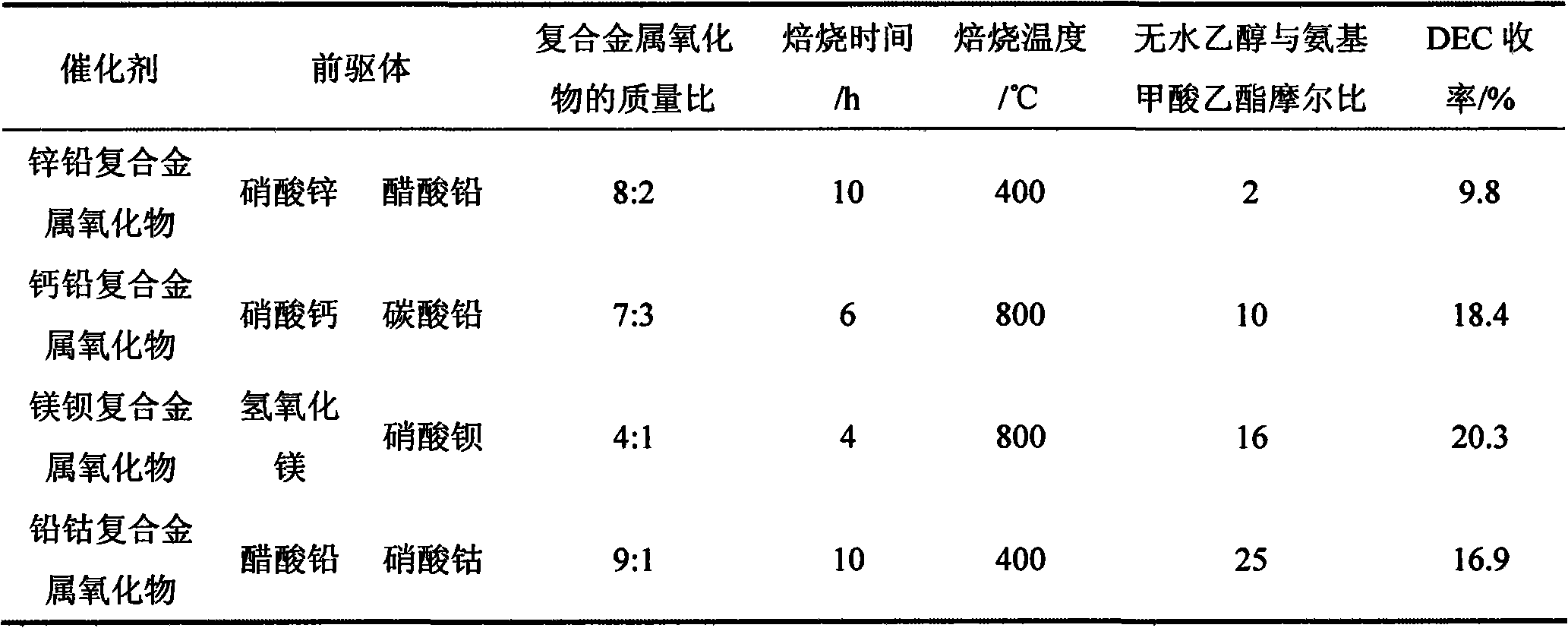
[0031] Table 2 The preparation conditions of different composite metal oxide catalysts and the influence of the molar ratio of absolute ethanol and ethyl carbamate on the performance of the catalyst
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com