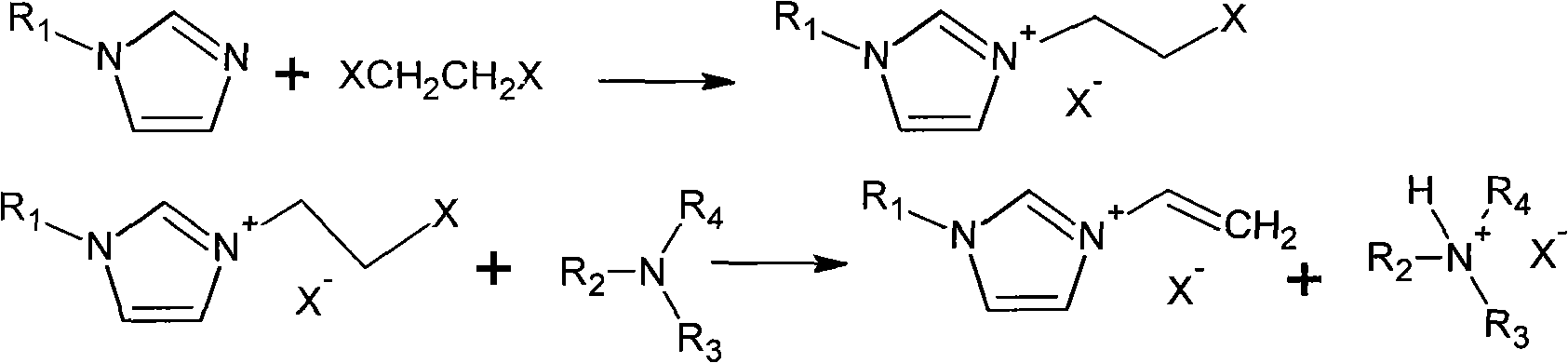
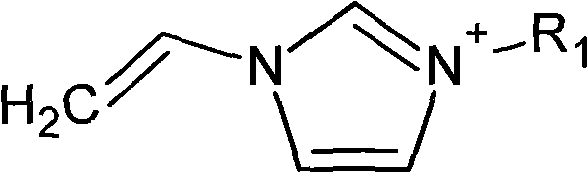
Method for preparing vinylimidazole ionic liquid
A vinylimidazole, ionic liquid technology, applied in the direction of organic chemistry and the like, can solve the problems of limited application and high price, and achieve the effects of novel method, low price and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Synthesis of 1-methyl-3-vinyl imidazolium chloride ionic liquid:
[0016] (1) Weigh 20 g (0.2021 mol) of 1,2-dichloroethane in a three-necked flask, and drop a mixture of 13.6942 g (0.1668 mol) of N-methylimidazole and 20 ml of acetone into the mixture through a constant pressure dropping funnel. In the three-necked flask, under vigorous stirring, the temperature was controlled at 50°C. After 20 hours of reaction, a large amount of white powder was produced, which was filtered, and the filter cake was washed several times with acetone and dried to obtain the product 1-methyl-3-(2 -Chloroethyl) imidazolium chloride 29g.
[0017] (2) Weigh 4g (0.0221mol) of 1-methyl-3-(2-chloroethyl)imidazolium chloride, 15g ethanol, 3.0308g (0.0300mol) of triethylamine, place in a single-necked flask, and stir vigorously , the temperature was controlled at 60°C, and after 12 hours of reaction, the solution turned into a light yellow transparent solution. After cooling at a low temperatu...
Embodiment 2
[0019] Synthesis of 1-ethyl-3-vinyl imidazolium chloride ionic liquid:
[0020] (1) Weigh 19.7940g (0.2mol) of 1,2-dichloroethane in a three-necked flask, and drop the mixture of 15.9526g (0.1660mol) of N-ethylimidazole and 20ml of acetone through a constant pressure dropping funnel. Put it into a three-necked flask, stir vigorously, and control the temperature at 50°C. After 20 hours of reaction, a large amount of white powder is produced, which is filtered, and the filter cake is washed several times with acetone, and dried to obtain the product 1-ethyl-3-(2 -Chloroethyl) imidazolium chloride 29.8976g.
[0021] (2) Weigh 4g (0.0205mol) of 1-ethyl-3-(2-chloroethyl) imidazolium chloride, 15g ethanol, 3.0534g (0.0302mol) of triethylamine, place in a single-necked flask, and stir vigorously , kept at 60°C, after 12 hours of reaction, it was a light yellow transparent solution, after standing for 5 hours, a large number of white needle-like crystals precipitated, filtered, and t...
Embodiment 3
[0023] Synthesis of 1-methyl-3-vinyl imidazolium bromide ionic liquid:
[0024] (1) Weigh 37.5761g (0.2mol) of 1,2-dibromoethane in a three-necked flask, and drop the mixture of N-methylimidazole 13.6987g (0.1669mol) and 20ml of acetone through a constant pressure dropping funnel Put it into a three-necked flask, under vigorous stirring, the temperature is controlled at 50 ° C, after 20 hours of reaction, a large amount of white powder is produced, which is filtered, the filter cake is washed several times with acetone, and dried to obtain the existing product 1-methyl-3-(2 -Chloroethyl) imidazolium bromide 42.5695g.
[0025] (2) Weigh 4 g (0.0148 mol) of 1-methyl-3-(2-chloroethyl) imidazolium bromide, 15 g ethanol, and 1.6165 g (0.0167 mol) of triethylamine, and place them in a single-necked flask under vigorous stirring , kept at 60°C, after 12 hours of reaction, it turned into a light yellow transparent solution, after standing for 5 hours, a large number of white needle-l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com