Pharmaceutical dosage forms for controlled release producing at least a timed pulse
A technology for pharmaceutical dosage forms and tablets, applied in the field of controlled release dosage forms, can solve problems such as the release speed is not fast enough and the drug cannot be released.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
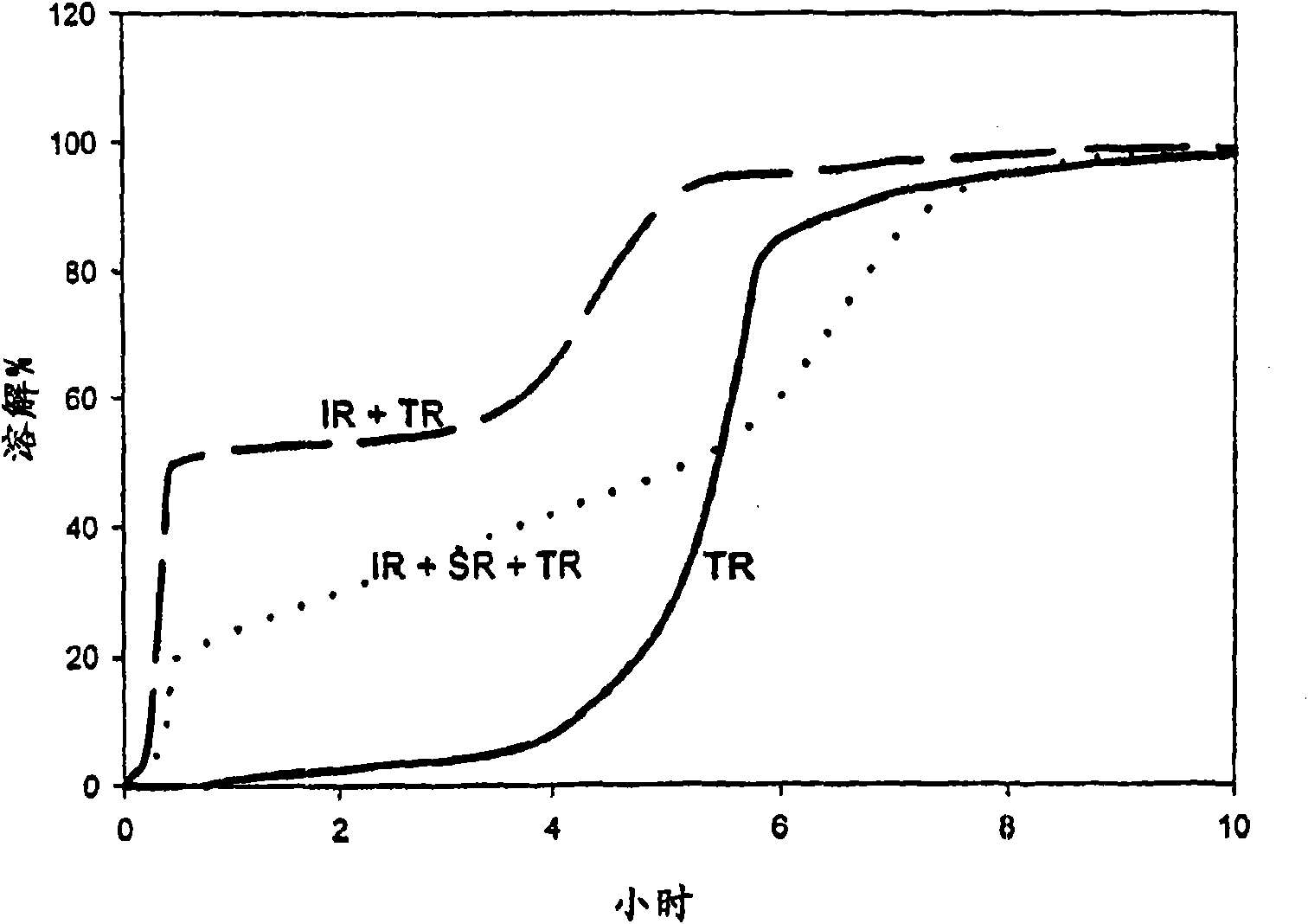
[0086] Example 1: Capsules Containing Alfuzosin Hydrochloride and Cetylpyridinium Chloride - Slow Release After Long Time Interval
[0087] Alfuzosin hydrochloride was loaded onto 3325 g of 16 / 18 mesh non-pareil beads by coating on a GPCG3 fluid bed coater-dryer with the suspension under the following conditions.
[0088] Alfuzosin Hydrochloride
5.0%
87.5g
5.0%
87.5g
90.0%
1575g
[0089] 1 Mowiol 5- Sold by Chimidis Hoechst
[0090] 1100 g of these alfuzosin-coated beads were then coated on a GPCG1 fluid bed coater-dryer with a suspension of the following composition:
[0091] cetylpyridinium chloride
4.3%
43.4g
4.7%
46.9g
Hydroxypropylmethylcellulose 2
5.9%
59.0g
42.5%
425.0g
Isopropanol
42.5%
425.0g
[0092] 2 Pharmacoat Sold by Sh...
Embodiment 2
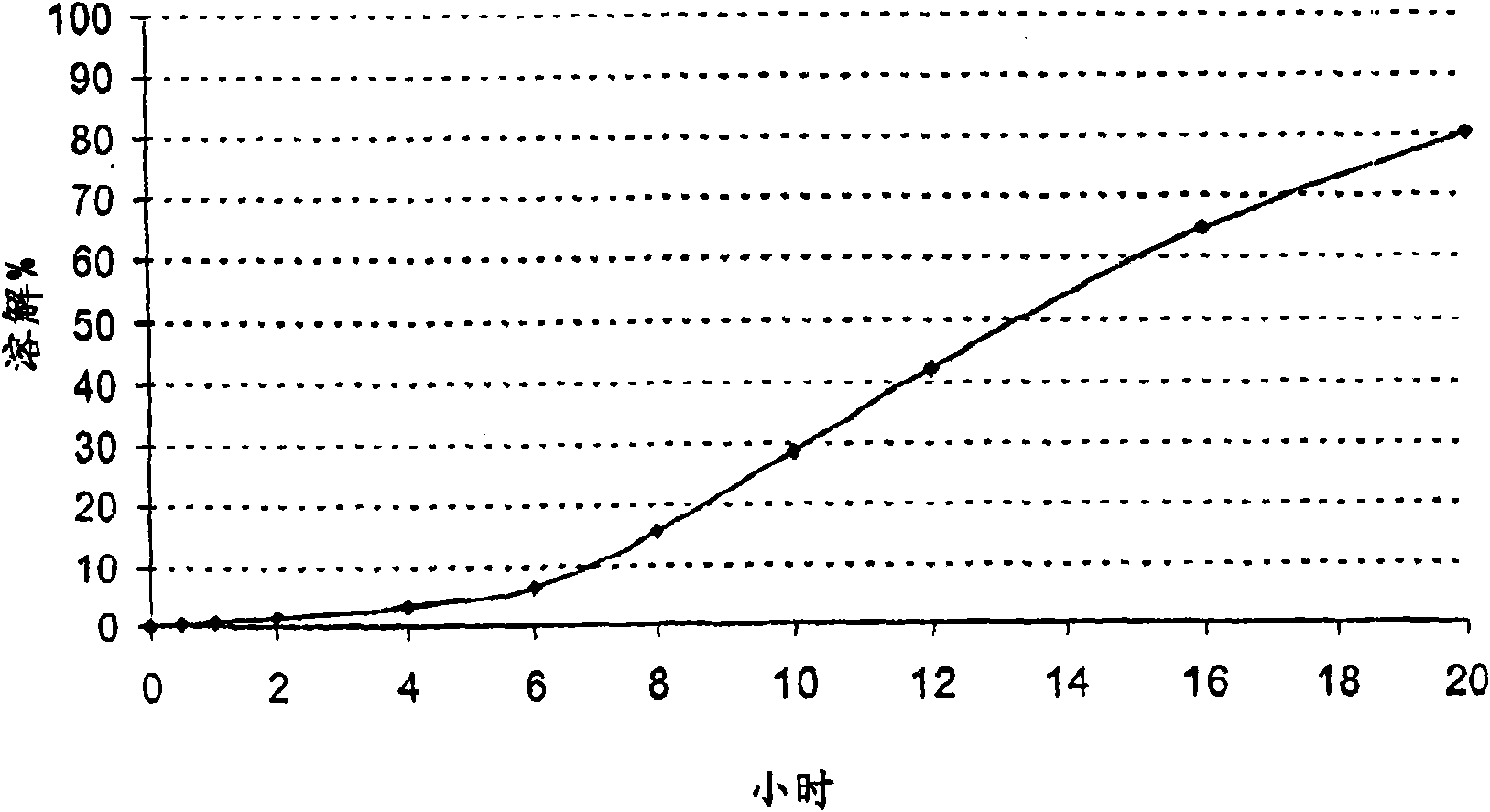
[0105] Embodiment 2: coated pellet
[0106] Delayed release pellets containing alfuzosin hydrochloride, tartaric acid and cetylpyridinium chloride and cationic surfactant.
[0107] Coat 1000g of 16 / 18 mesh Super Beads with a suspension composed as follows:
[0108] tartaric acid
6.0%
78.0g
Hydroxypropylmethylcellulose 1
4.0%
53.0g
3.0%
39.0g
1.4%
18.2g
43.8%
557g
Isopropanol
43.8%
557g
[0109] 1 Pharmacoat Sold by Shin-Etsu
[0110] Then in the GPCG1 fluidized bed coater-dryer, coat with the following solution to make alfuzosin hydrochloride be loaded on the pellets:
[0111] Alfuzosin Hydrochloride
8.3%
78g
povidone 2
8.3%
78g
83.4%
784g
[0112] Sold by BASF
[0113] Finally, 1000 g of the above-mentioned pelle...
Embodiment 3
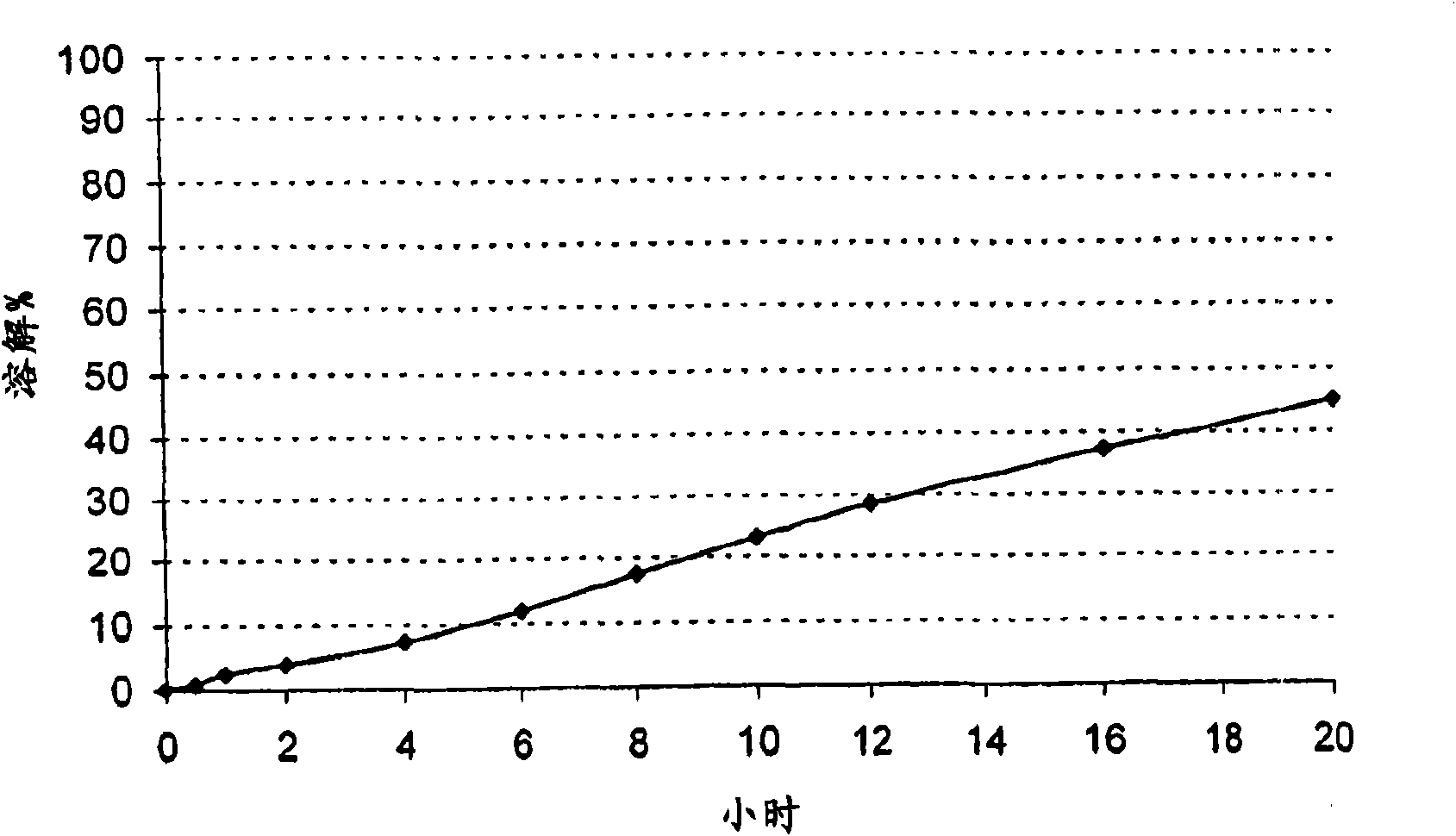
[0118] Embodiment 3: Coated pellets:
[0119] Delayed release pellets containing alfuzosin hydrochloride, succinic acid and cocamidopropyl betaine zwitterionic surfactants.
[0120] Coat 1000g of 16 / 18 mesh Super Beads with a suspension composed as follows:
[0121] Succinic acid
5.63%
78.0g
Hydroxypropylmethylcellulose 1
3.82%
53.0g
2.81%
39.0g
purified water
43.87%
608g
Isopropanol
43.87%
608g
[0122] 1 Pharmacoat Sold by Shin-Etsu
[0123] 380LC sold by Seppic
[0124] Alfuzosin hydrochloride was then loaded onto the pellets as described in Example 2:
[0125] Finally, 1000 g of the above-mentioned pellets were coated with a polymer solution of the following composition:
[0126] Type B ammonium methacrylate copolymer 3
11.40%
208.5g
Type A Ammonium Methacrylate Copolymer 4
0.93%
17g
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com