Erythrocyte generating characteristic-free erythropoietin with nervous protecting function and application thereof
A technology of erythropoietin and production characteristics, applied in new erythropoietin and its pharmaceutical field, can solve the problems of losing or losing the function of stimulating erythropoiesis, so as to increase safety, avoid secondary thrombosis, expand Effects of treatment groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Site-directed mutagenesis of EPO cDNA
[0054] Human EPO cDNA was amplified from the human kidney cDNA library, and 62 new mutant EPO cDNAs were obtained by site-directed mutation by PCR method, and these mutated EPO cDNAs were loaded into eukaryotic expression vectors. The detailed steps are as follows:
[0055] 1. Amplification of human EPO cDNA
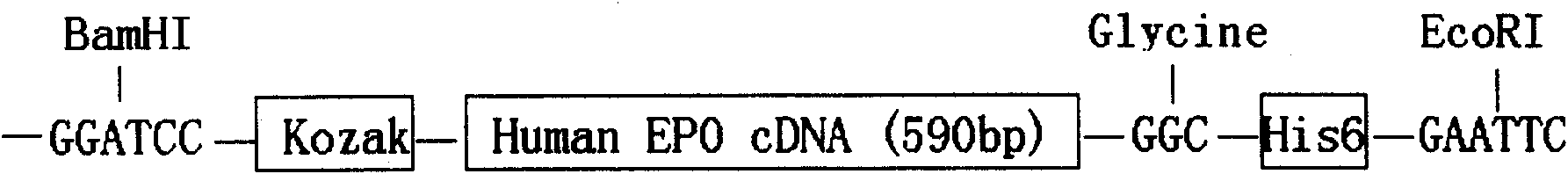
[0056] Human EPO cDNA encodes 193 amino acids. After the EPO primary protein is synthesized, the signal peptide containing 27 amino acid residues at the amino terminal becomes mature EPO after cleavage. Mature human EPO is thus 166 amino acids. Based on the above research results, a histidine tag (His-6) for isolation and purification was added to the 3' end of EPO cDNA. Human EPO cDNA containing the His-6 tag was amplified by PCR from a human kidney Marathon-ready cDNA library (Clontech, Mountain View, USA). Primers were designed according to the published sequence of human EPO cDNA (gene sequence number: NM00...
Embodiment 2
[0188] Example 2: Preparation and purification of mEPO fusion protein
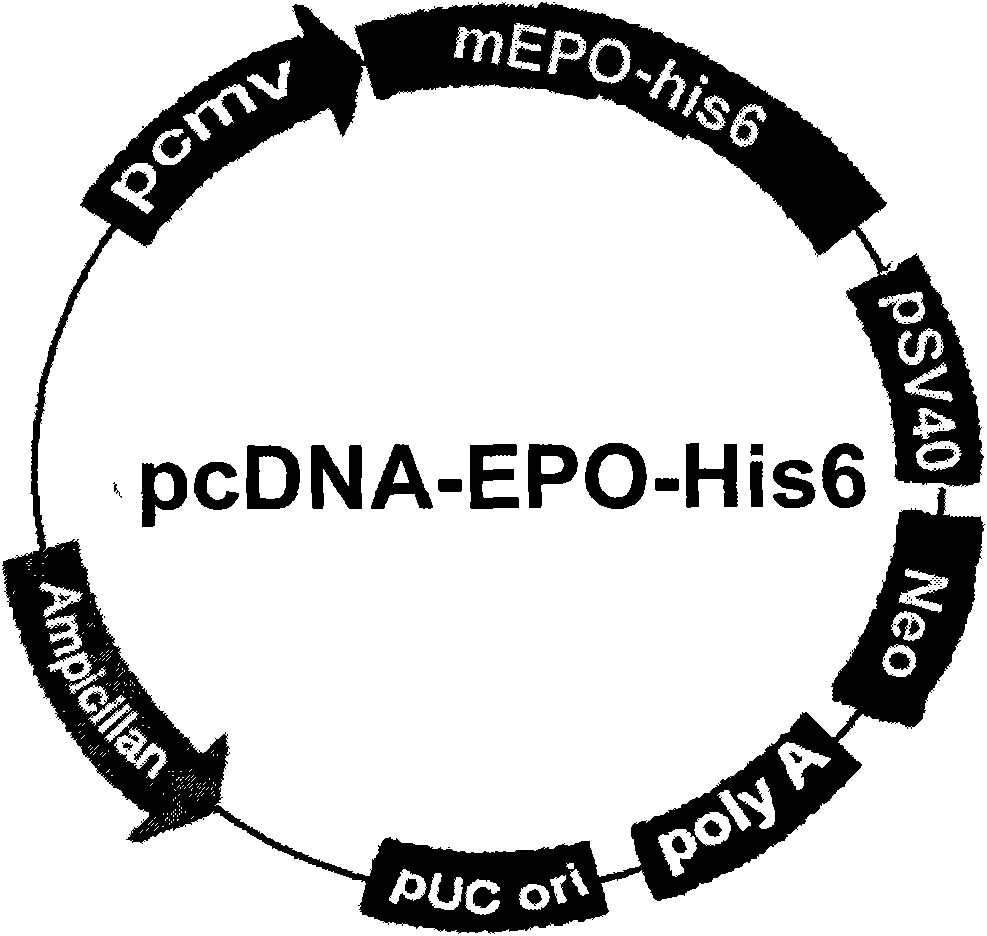
[0189] The vector pcDNA-mEPO-his6 was introduced into 293 cell line with transfection reagent Lipofectamine 2000 (Invitrogen, USA). After 24 hours of transfection, subculture at 1:12, add G4 181 mg / ml (Invitrogen, USA) for culture for 2 weeks, and then select a single clone and transfer it to a 6-well plate for further culture. Take 10 μl of medium and use Western blot or ELISA to identify cell lines with high expression of mEPO after diluting 1000 times. The expression of EPO was identified by Western blot and ELISA in the supernatant, the results are shown in figure 2. After a large amount of mEPO high-expressing cell lines were amplified, the culture medium was collected and purified by Ni-NTA agarose chromatography column (QIAGEN, Valencia, USA) to recover and purify the mEPO fusion protein. Briefly, the medium was adjusted to pH 8.0 with NaOH and 5M NaCl was added to a final concentration of 300 m...
Embodiment 3
[0191] Example 3: Detecting whether mEPO has erythropoietic properties
[0192] We investigated whether these mEPOs have erythropoietic properties using in vivo and in vitro experiments.
[0193] 1. In vitro analysis of biological characteristics of mEPO erythropoiesis
[0194] 32D-EPOR cells are cell lines formed after the lymphoblastoid 32D cells derived from mouse bone marrow are stably transfected with EPO receptors. analyze. 32D-EPOR cells were seeded in six-well plates with IDEM medium (Invitrogen), about 2×10 per well 5 cells. After 6 hours of starvation (without the presence of EPO), wild-type EPO (1 U / ml) or mEPO (1 and 100 U / ml) or PBS (control) were added, and stained with placenta blue after 72 hours, the number of viable cells was counted. In the presence of wild-type EPO, 32D-EPOR cells proliferated approximately 13-fold (2.7x10 7 ), while mEPO was the same as the PBS control, only 0.1% of the cells survived, even after increasing the concentration of mEPO b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com