Pharmaceutical composition for treating hyperplasia diseases
A technology for proliferative diseases and compositions, applied in the field of pharmaceutical compositions containing a compound of formula I or a pharmaceutically acceptable salt thereof combined with an anti-proliferative agent, capable of solving problems such as no tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
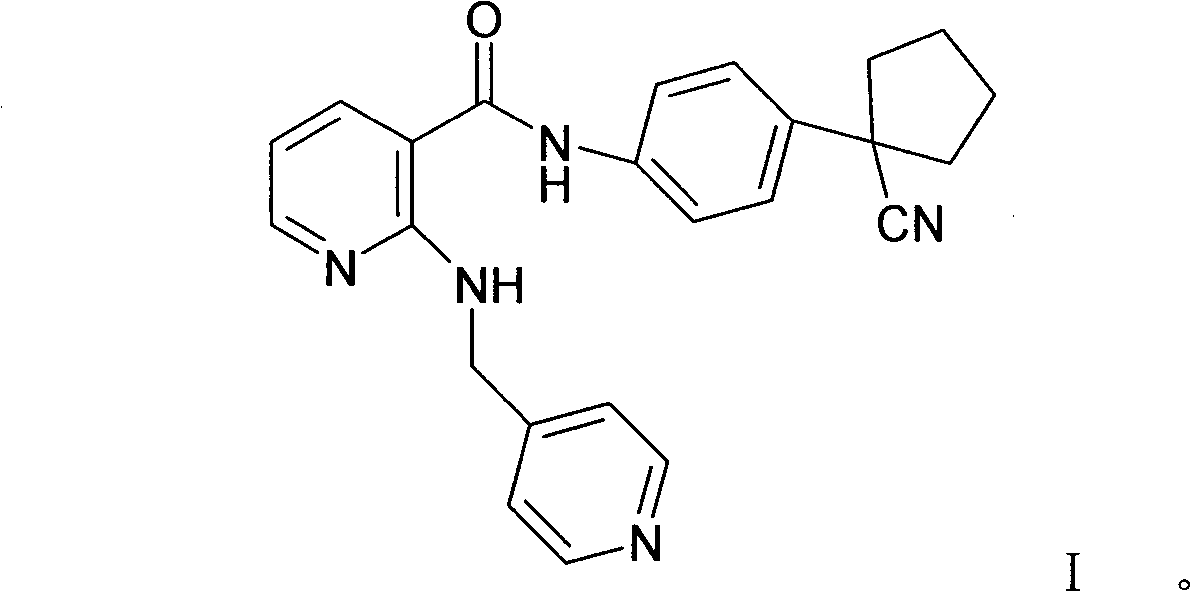
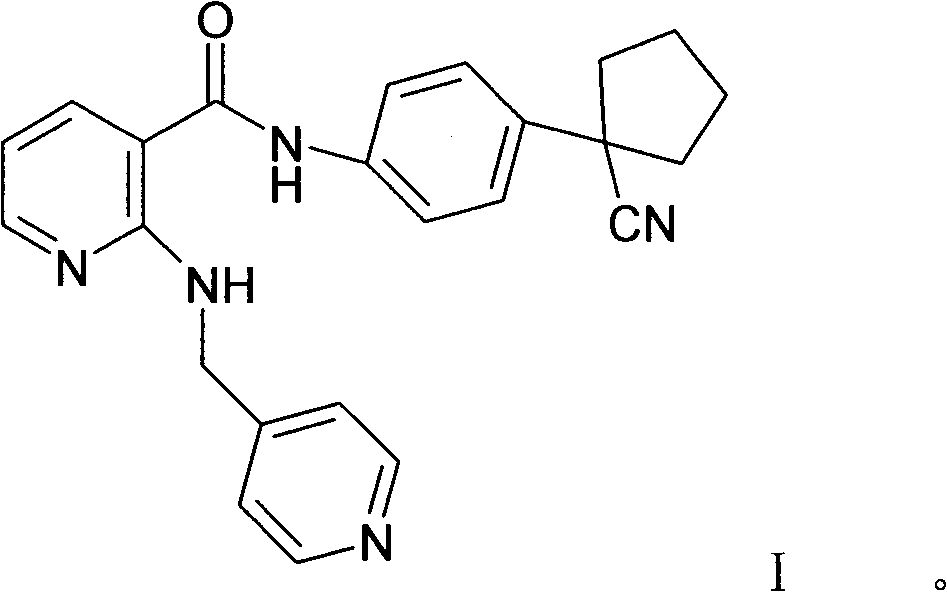
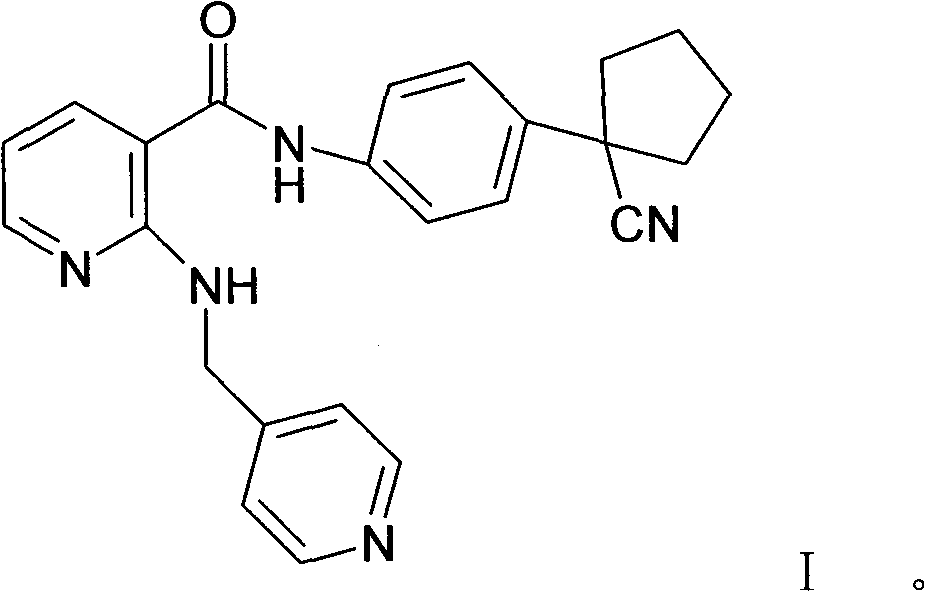
[0087] Embodiment 1: preparation compound A (the mesylate of formula I compound)
[0088] In a 5L reaction flask, put 170g (0.428mol) of the compound of formula I, 42.5g (0.442mol) of methanesulfonic acid, 2.55L of 95% isopropanol aqueous solution, stir and heat under nitrogen protection and light-shielding conditions until completely dissolved, to obtain The light yellow transparent solution was filtered while it was hot, cooled and crystallized to room temperature, filtered, washed with isopropanol, and dried in vacuum to obtain 180.2 g (0.365 mol) of white needle-like crystals, with a yield of 85.4%.
[0089]In a 5L reaction flask, put 180.2g of compound A, 2.52L of 95% isopropanol aqueous solution, stir and heat until completely dissolved under nitrogen protection and dark conditions, filter while hot, and cool the filtrate to room temperature for crystallization, filter, isopropanol Wash and dry in vacuo to obtain 161.5 g of white needle-like crystals, with a yield of 89...
Embodiment 2
[0090] Embodiment 2: Compound A is used alone or combined with oxaliplatin for the treatment of human colon cancer Ls174t nude mouse transplanted tumor effect
[0091] Explanation: Compound A is the methanesulfonate of the compound of formula I, the same below.
[0092] 1. Experimental animals:
[0093] BALB / cA-nude nude mice, ♀, 5-6 weeks old, were purchased from Shanghai Slack Experimental Animal Co., Ltd. Certificate number: SCXK (Shanghai) 2004-0005. Breeding environment: SPF grade.
[0094] 2. Experimental steps:
[0095] After 1 week of adaptation, the animals were subcutaneously inoculated with human colon cancer Ls174t tumor tissue until the tumor grew to 150-300mm 3 Afterwards, the animals were randomly divided into groups (d0) for administration. Compound A and PTK787 are both 75mg / kg, administered orally (gavage), d0-d13, once a day, a total of 14 times; oxaliplatin 6mg / kg, intravenous injection, d0, d4, d8, a total of 3 times . When used in combination, ...
Embodiment 3
[0102] Embodiment 3: The curative effect of compound A alone or combined with 5-Fu on human colon cancer Ls174t nude mice xenografts
[0103] 1. Experimental animals:
[0104] BALB / cA-nude nude mice, ♀, 5-6 weeks old, were purchased from Shanghai Slack Experimental Animal Co., Ltd. Certificate number: SCXK (Shanghai) 2004-0005. Breeding environment: SPF grade.
[0105] 2. Experimental steps:
[0106] After 1 week of adaptation, the animals were subcutaneously inoculated with human colon cancer Ls174t tumor tissue until the tumor grew to 150-300mm 3 Afterwards, the animals were randomly divided into groups (d0) for administration. Both compound A and PTK787 were given 75 mg / kg orally (gavage), d0-d13, once a day, 14 times in total; 5-Fu 50 mg / kg, intraperitoneally injected, d0, d4, d8, 3 times in total. When used in combination, compound A and PTK787 were used in combination with 5-Fu respectively, and the dosage and regimen remained unchanged. The tumor volume was meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com