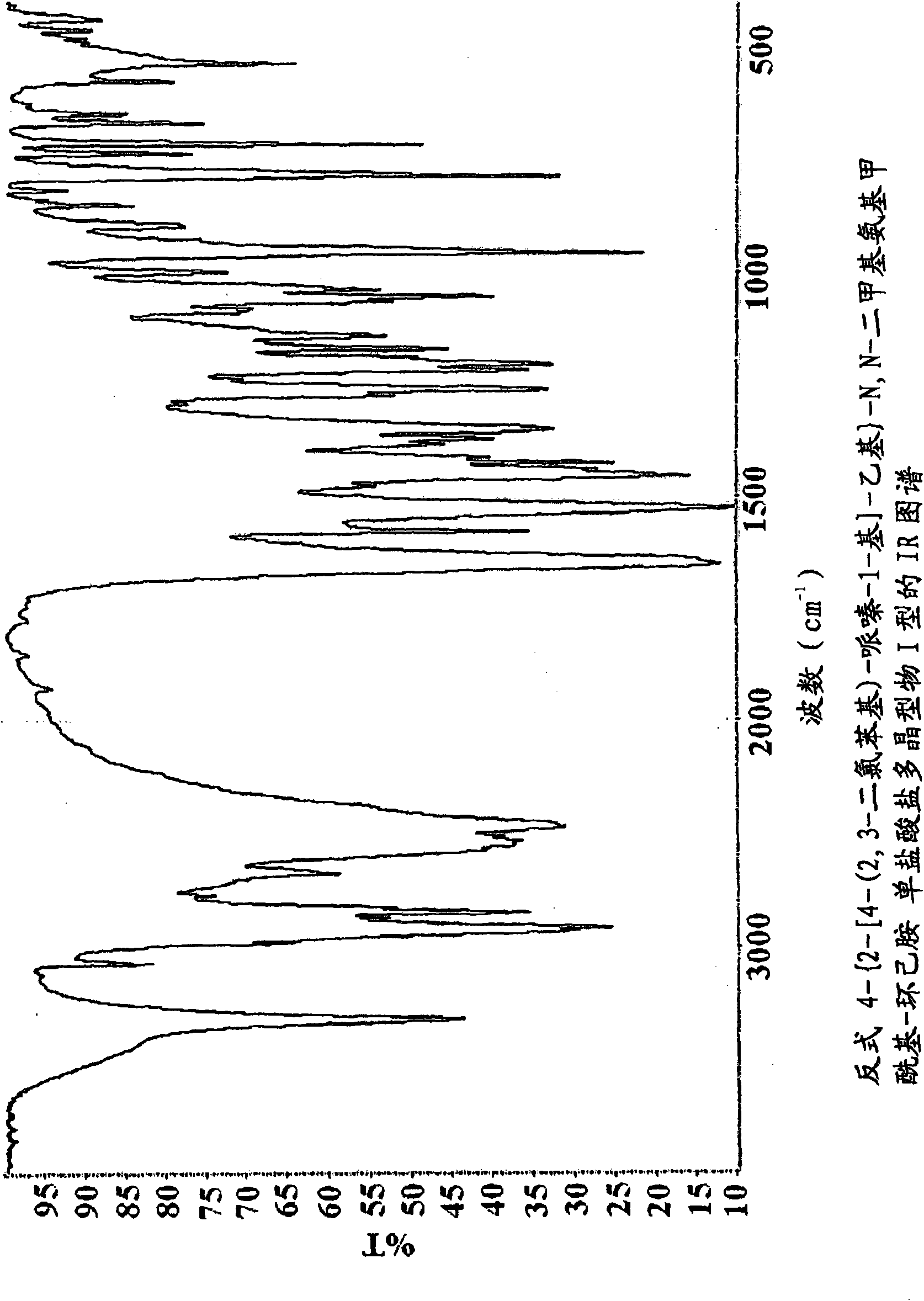
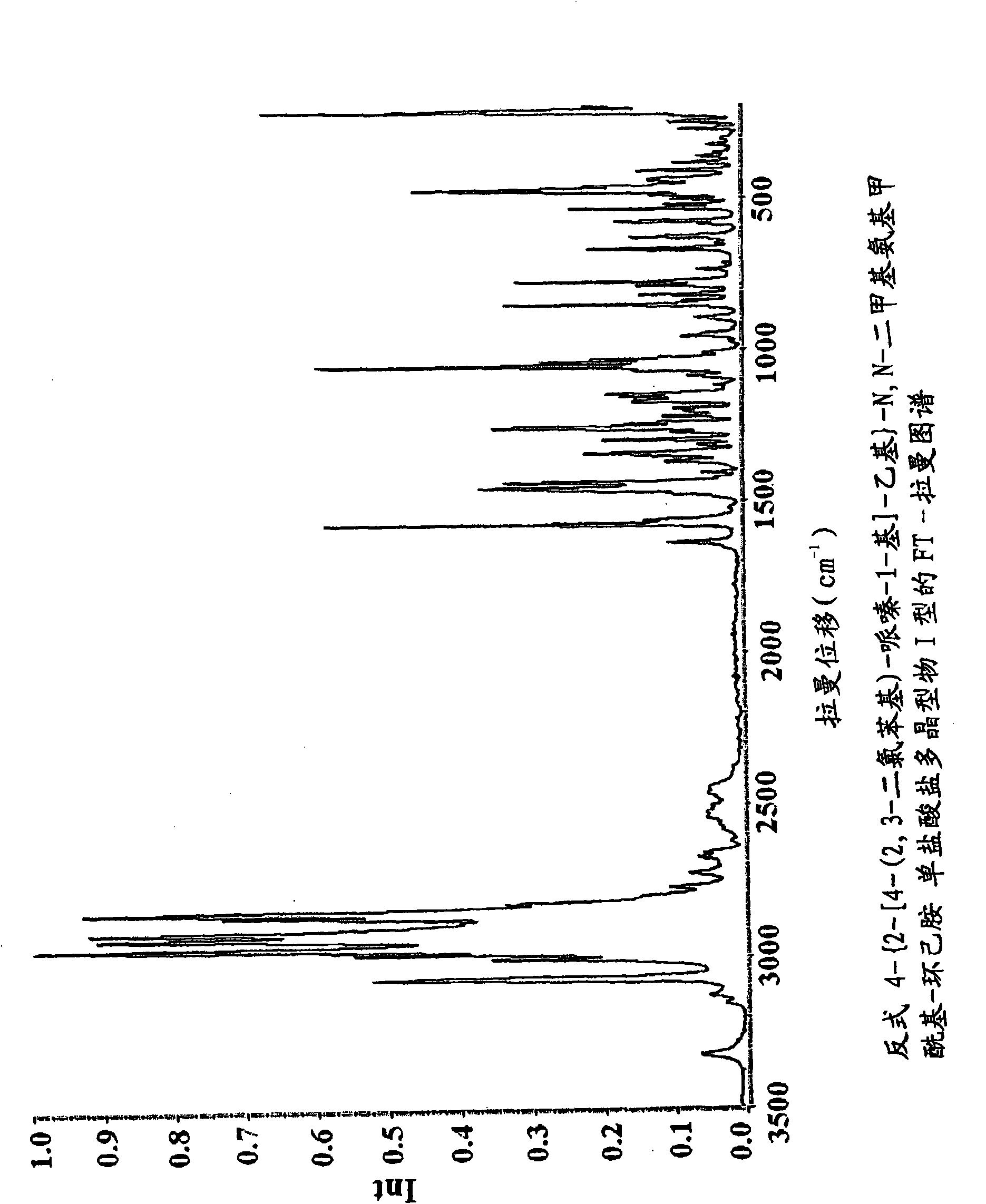
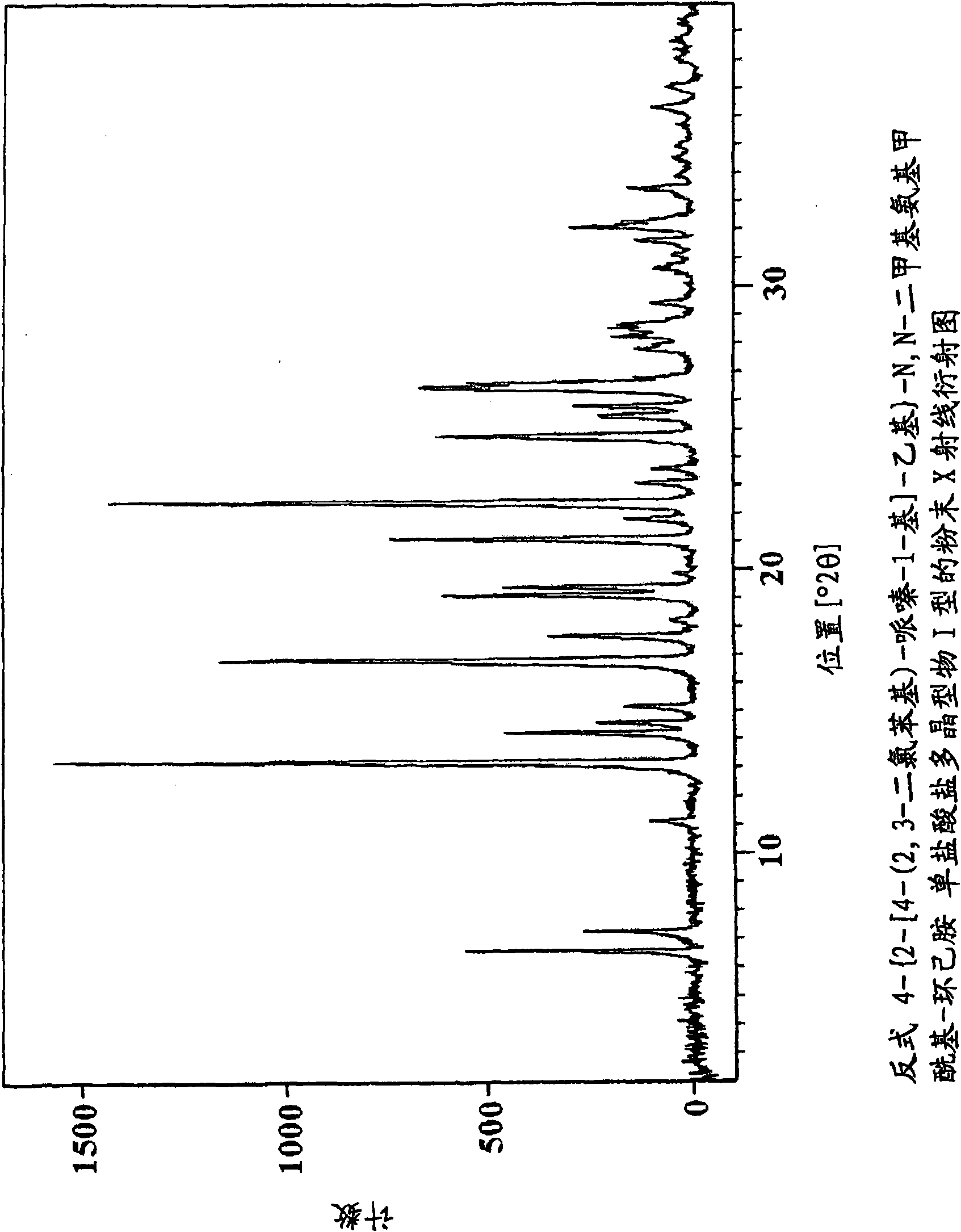
Novel piperazine salts as d3/d2 antagonists
A kind of technology of piperazine and solvate, applied in the field of novel piperazine salt as D3/D2 antagonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] trans 4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]ethyl}-N,N-dimethylcarbamoyl-cyclohexylamine mesylate
[0038] 3.0g (0.007mol) of trans 4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]ethyl}-N,N-dimethylcarbamoyl - Cyclohexylamine and 0.46 ml (0.007 mol) of methanesulfonic acid are mixed in a mixture of 10 ml methanol and 80 ml acetonitrile. The reaction mixture was heated to boiling temperature, and the homogeneous solution obtained was concentrated to 25 ml by distillation. The resulting suspension was then stirred at a temperature of 0-5°C for 2 hours and the product was isolated by filtration.
[0039] In this way, 3.1 g of the title compound were obtained.
[0040] Yield: 84%.
[0041] Melting point: 225-229°C.
Embodiment 2
[0043] trans 4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]ethyl}-N,N-dimethylcarbamoyl-cyclohexylamine maleate
[0044] 3.0g (0.007mol) of trans 4-{2-[4-(2,3-dichlorophenyl-piperazin-1-yl]ethyl}-N,N-dimethylcarbamoyl- Cyclohexylamine and 0.83g (0.007mol) of maleic acid are suspended in 150ml acetone.The reaction mixture is heated to boiling temperature and stirred for half an hour, then distillation is concentrated to 25ml.Then the suspension obtained is at 0-5 The temperature was stirred for 2 hours at a temperature of °C, and the product was isolated by filtration.
[0045] In this way, 3.14 g of the title compound were obtained.
[0046] Yield: 82%.
[0047] Melting point: 173-177°C.
Embodiment 3
[0049] trans 4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]ethyl}-N,N-dimethylcarbamoyl-cyclohexylamine monohydrobromide Salt
[0050] 3.0g (0.007mol) of trans 4-{2-[4-(2,3-dichlorophenyl-piperazin-1-yl]ethyl}-N,N-dimethylcarbamoyl- Cyclohexylamine is suspended in the mixture of 12ml methyl alcohol and 38ml (1.5%) hydrobromic acid solution.Reaction mixture is heated to boiling point temperature and the homogeneous solution that obtains is cooled to the temperature of 0-5 ℃ and lasted 1 hour, then in After stirring at the same temperature for 2 hours, the product was isolated by filtration.
[0051] In this way, 3.0 g of the title compound were obtained.
[0052] Yield: 85%.
[0053] Melting point: 248-252°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com