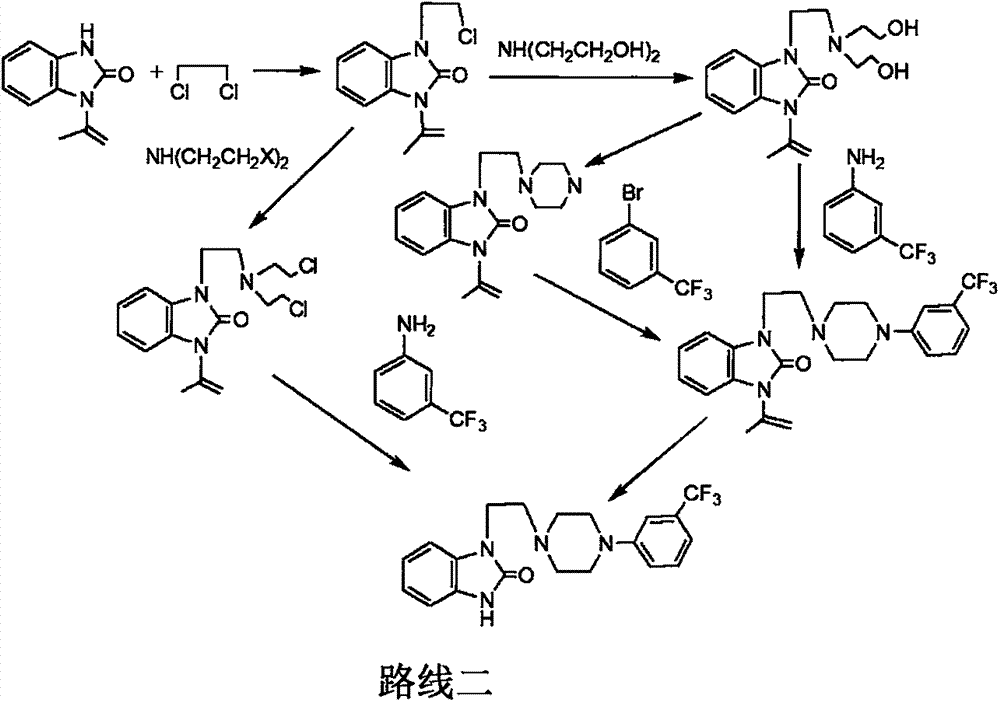
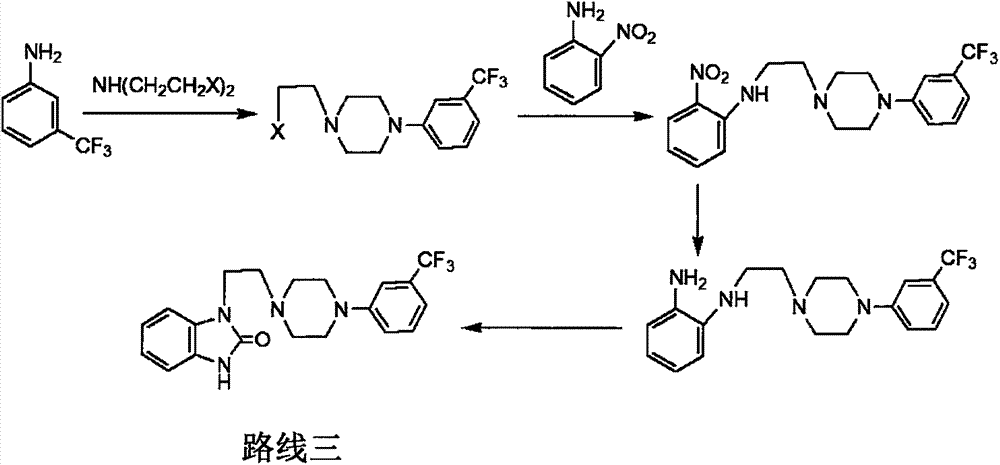
Novel process for preparing flibanserin
A technology of flibanserin and new technology, which is applied in the field of new technology for the preparation of drug flibanserin, and can solve the problems of difficult industrialization, unsimplified and cumbersome reaction process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
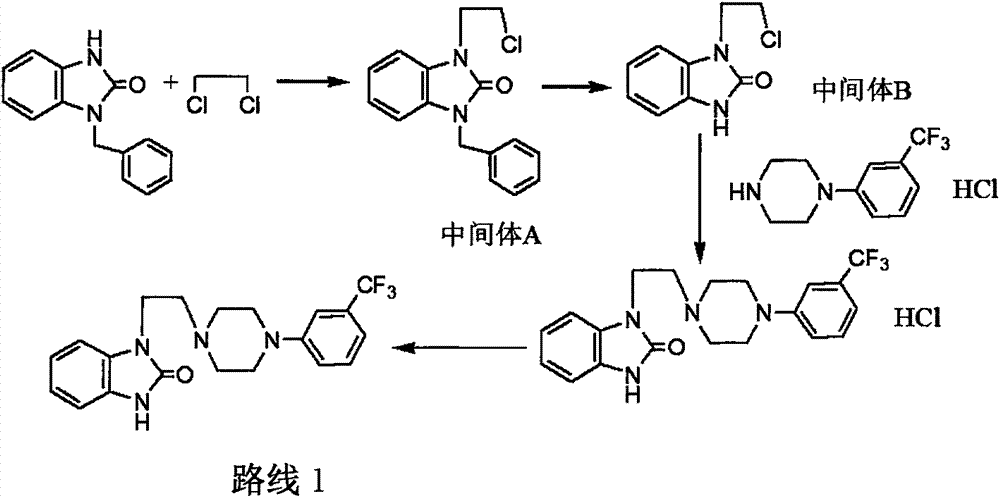
[0031] Preparation of Intermediate 1
[0032] Add 1-benzyl-2-benzimidazolone (10 g, 0.044 mol) into 50 mL of dehydrated DMF, cool in an ice bath to 10 ° C, control the temperature below 20 ° C and slowly add 2 g (60%) of sodium hydride, add Stir at room temperature for 3 hours, cool to below 5°C and add 1,2-dibromoethane (16.5 g, 0.088 mol) dropwise, return to room temperature and stir for 3 hours, then pour the reaction solution into ice water and extract with dichloromethane. The extract was washed with water, dried, filtered and evaporated to dryness to obtain 10.8 g of the product with a yield of 73%.
Embodiment 2
[0034] Preparation of Intermediate 1
[0035] Add 1-benzyl-2-benzimidazolone (10g, 0.044mol) into 50mL of DMF, cool in an ice bath to 10°C, control the temperature below 20°C and slowly add 5g of potassium hydroxide, and stir at room temperature for 3 Hours later, 1,2-dibromoethane (16.5 g, 0.088 mol) was added dropwise and stirred at room temperature for 3 hours. The reaction solution was poured into ice water, extracted with dichloromethane, the extract was washed with water, dried, filtered and evaporated to dryness to obtain Product 8.9g, yield 60%.
Embodiment 3
[0037] Synthesis of Intermediate 2
[0038] Intermediate 1 (30g, 0.0905mol) and 1-(3-trifluoromethylphenyl)piperazine salt (24.1g, 0.0905mol) were added to 200mL acetone, stirred at room temperature for 15 minutes, and 20g of potassium hydroxide was added Raise the temperature to reflux, react for 12 hours, cool down to room temperature, put the reaction solution into 1L of water, add 300mL of dichloromethane to extract twice, combine the organic layers, dry over sodium sulfate, filter, evaporate the filtrate to dryness, add 300mL of methanol and water mixture, Stir at room temperature for 3 hours, filter with suction, and dry the product at room temperature to obtain 27.9 g of the product with a yield of 64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com