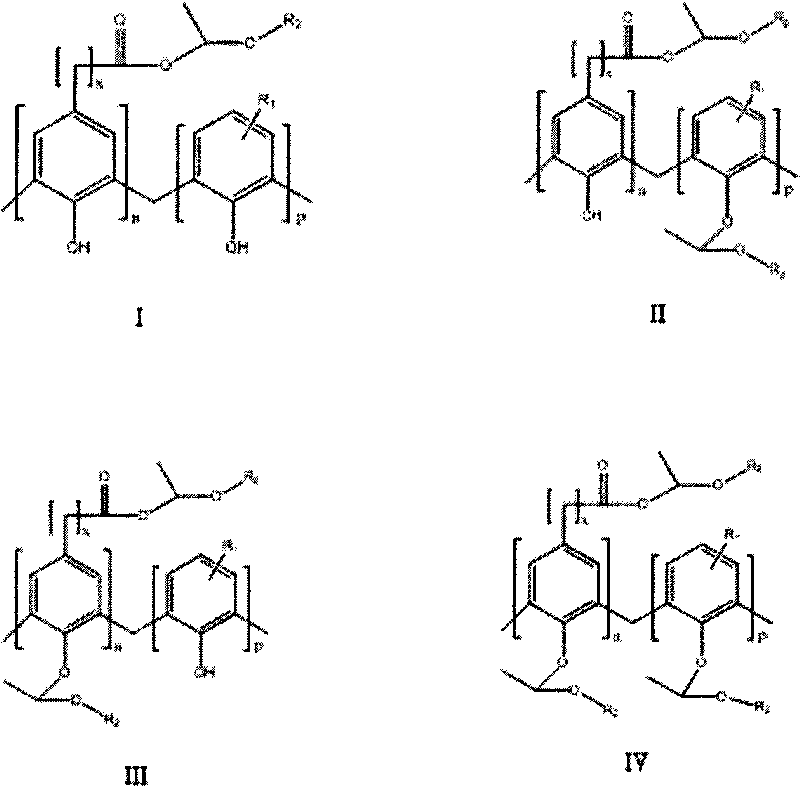
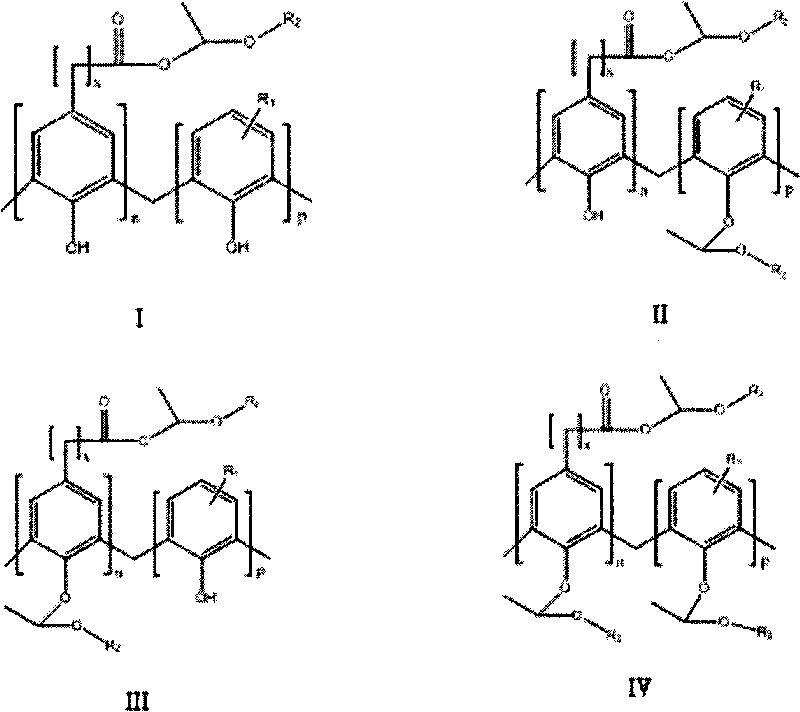
Synthesis of carboxylic phenol resin active ester and etherate
A technology of phenolic resin and active ester, applied in the field of polymer material preparation, can solve the problems of difficulty in storage, poor compound stability, influence on printing endurance, etc., and achieve the effects of excellent comprehensive performance, high etherification rate and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 13g of carboxyl phenolic resin with code DF-10, 100mL of ether, 1.00g of phosphoric acid into a four-necked flask equipped with a stirrer, condenser, constant pressure dropping funnel and thermometer, and add 100ml of ethylene dropwise with a constant pressure dropping funnel Diethyl ether, after the dropwise addition, reacted at room temperature for 6 hours, washed with water, and dried in vacuo to obtain 12.6 g of a yellow solid, that is, carboxyl phenolic resin active ester and etherified product.
Embodiment 2
[0020] Add 5g carboxyl phenolic resin with code DF-10, 100mL 1,2-dichloroethane, 0.5g acetonyl triphenyl For phosphine bromide, add 60ml of vinyl ether dropwise with a constant pressure dropping funnel. After the dropwise addition, react at room temperature for 6 hours, wash with water, and dry in vacuo to obtain 5.1g of yellow solid, which is carboxyphenolic resin active ester and etherified product.
Embodiment 3
[0022] Add 6g of carboxyl phenolic resin whose code is DF-10, 100mL of dichloromethane, and 0.2g of acetonyl triphenylphosphine bromide to a four-necked flask equipped with a stirrer, condenser, constant pressure dropping funnel, and thermometer. Add 70ml of vinyl ethyl ether dropwise into the constant pressure dropping funnel. After the dropwise addition, react at room temperature for 3 hours, wash with water, and dry in vacuum to obtain 6.3g of yellow solid, which is carboxyphenolic resin active ester and etherified product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com