Method for extracting palladium from high-level liquid waste
A high-level waste liquid extraction technology, applied in the direction of improving process efficiency, can solve the problems of low distribution ratio, long extraction time, poor stability of extraction agent, etc., and achieve the effect of minimizing waste and simplifying process equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In this embodiment, a palladium nitric acid solution is used to simulate a high-level waste liquid to carry out a single-stage experiment of extracting palladium. The specific steps are as follows:
[0032] a) prepare palladium solution, wherein C Pd =200 μg / mL, C HNO3 =2mol / L.
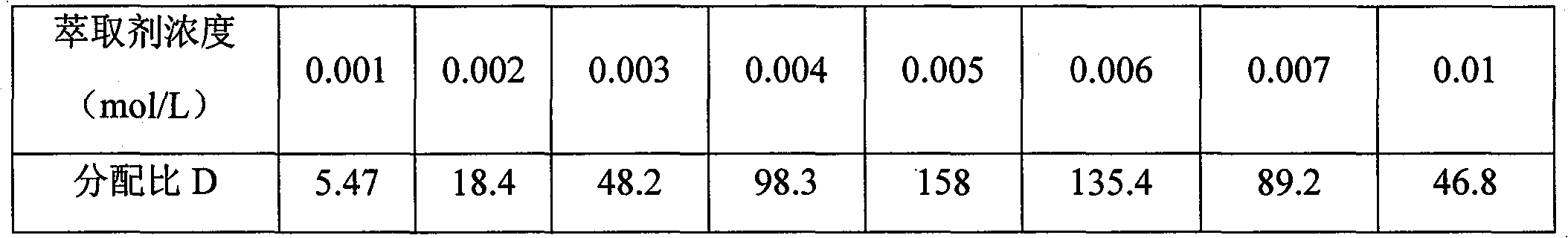
[0033] b) prepare TiBPS-xylene solution, wherein C TIBPS = 0.005 mol / L.
[0034] c) The palladium solution and the TiBPS-xylene solution with a ratio of 1:1 were mixed and vortexed, the operating temperature was room temperature, and the extraction time was 15 min.
[0035] d) After the extraction is balanced, the organic phase is separated from the water phase, and the concentration of palladium in the water phase is analyzed to calculate the extraction distribution ratio.
[0036] e) Washing the extract phase to remove impurities.
[0037] f) adding 5% ammonia water to the washed extract phase for back extraction.
[0038] Experimental results: The extraction balance can be reached afte...
Embodiment 2
[0040] In this embodiment, a palladium nitric acid solution is used to simulate a high-level waste liquid to carry out a single-stage experiment of extracting palladium. The specific steps are as follows:
[0041] a) prepare palladium solution, wherein C Pd =200 μg / mL, C HNO3 = 1 mol / L.
[0042] b) prepare TiBPS-xylene solution, wherein C TIBPS = 0.004 mol / L.
[0043] c) The palladium solution and the TiBPS-xylene solution with a ratio of 1:1 were mixed and vortexed, the operating temperature was room temperature, and the extraction time was 15 min.
[0044]d) After the extraction is balanced, the organic phase is separated from the water phase, and the concentration of palladium in the water phase is analyzed to calculate the extraction distribution ratio.
[0045] e) Washing the extract phase to remove impurities.
[0046] f) adding 5% ammonia water to the washed extract phase for back extraction.
[0047] Experimental results: The extraction balance can be reached aft...
Embodiment 3
[0049] In this embodiment, a palladium nitric acid solution is used to simulate a high-level waste liquid to carry out a single-stage experiment of extracting palladium. The specific steps are as follows:
[0050] a) prepare palladium solution, wherein C Pd =200 μg / mL, C HNO3 = 3 mol / L.
[0051] b) prepare TiBPS-xylene solution, wherein C TIBPS = 0.006 mol / L.
[0052] c) The palladium solution and the TiBPS-xylene solution with a ratio of 1:1 were mixed and vortexed, the operating temperature was room temperature, and the extraction time was 15 min.
[0053] d) After the extraction is balanced, the organic phase is separated from the water phase, and the concentration of palladium in the water phase is analyzed to calculate the extraction distribution ratio.
[0054] e) Washing the extract phase to remove impurities.
[0055] f) adding 5% ammonia water to the washed extract phase for back extraction.
[0056] Experimental results: The extraction balance can be reached af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com