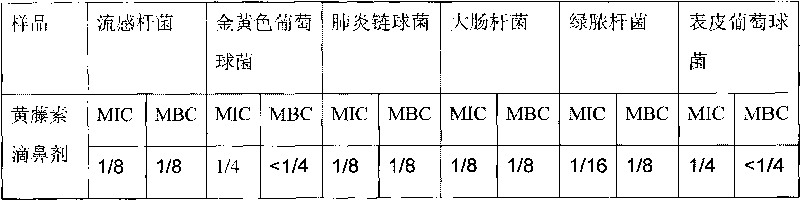
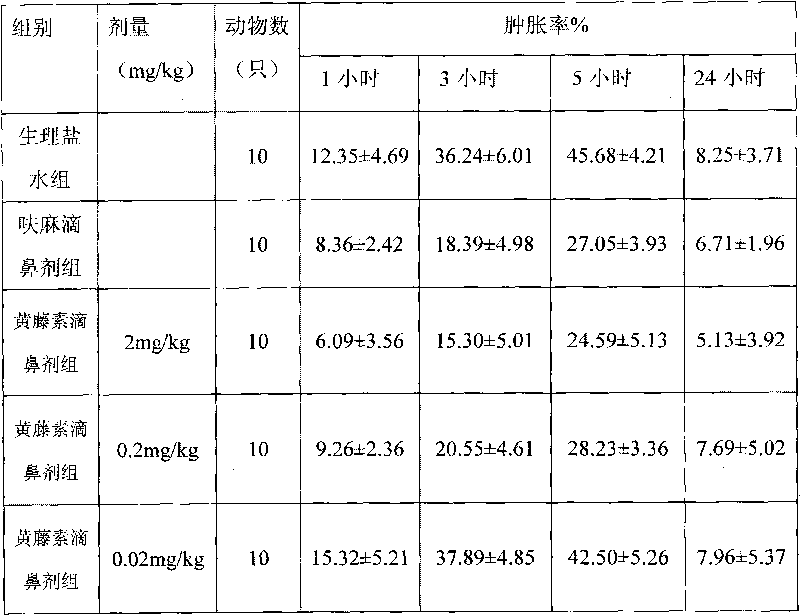
Nose drops taking fibrauretine as main medicine and preparation method
A kind of technology of patinarin and nasal drops, applied in the field of research and development of new pharmaceutical products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Drug formulation:
[0066] Palmatine 7mg Hydroxypropyl Methyl Cellulose 10g
[0067] 0.5mol / L NaOH solution ethylparaben 0.3g
[0068] 1000mL of normal saline with a concentration of 0.9% by mass
[0069] Preparation:
[0070] Weigh 7 mg of palmatine powder and 10 g of hydroxypropyl methylcellulose; dissolve the hydroxypropyl methylcellulose using the pulping method, take 250ml of hot water at 80-90°C to swell and disperse, and add hydroxypropyl methylcellulose Stir the base cellulose for 15 minutes to make it form a uniform insoluble paste, then stir it with an equal amount of room temperature water for 15 minutes to dissolve completely; add 600 mL of palmatine powder and ethyl paraben to normal saline, heat to dissolve, and Heat suction filtration to obtain the supernatant. Stir the hydroxypropyl methylcellulose solution and the palmatine supernatant evenly, add physiological saline to make the volume to 1000mL, stir well, adjust the pH value to 6.0 with an approp...
Embodiment 2
[0072] Drug formulation:
[0073] Palmatine 20mg Hydroxypropyl Methyl Cellulose 5g
[0074] 0.5mol / L NaOH solution ethylparaben 0.3g
[0075] 1000mL of normal saline with a concentration of 0.9% by mass
[0076] Preparation:
[0077] Weigh 20 mg of palmatine powder and 5 g of hydroxypropyl methylcellulose; dissolve the hydroxypropyl methylcellulose using the pulping method, take 200 ml of hot water at 80-90 ° C to swell and disperse, and add hydroxypropyl methylcellulose Stir the cellulose-based cellulose for 20 minutes to form a uniform insoluble paste, and then stir it with an equal amount of room temperature water for 20 minutes to completely dissolve it; add 600 mL of palmatine powder and ethyl paraben to normal saline, heat to dissolve, and Heat suction filtration to obtain the supernatant. Stir the hydroxypropyl methylcellulose solution and the palmatine supernatant evenly, add physiological saline to make the volume to 1000mL, stir well, adjust the pH value to 6.5 w...
Embodiment 3
[0079] Drug formulation:
[0080] Palmatine 1mg Hydroxypropyl Methyl Cellulose 15g
[0081] 0.5mol / L NaOH solution ethylparaben 0.3g
[0082] 1000mL of normal saline with a concentration of 0.9% by mass
[0083] Preparation:
[0084] Weigh 1 mg of palmatine powder, 15 g of hydroxypropyl methylcellulose; dissolve the hydroxypropyl methylcellulose by pulping method, take 300ml of hot water at 80-90°C to swell and disperse, and add hydroxypropyl methylcellulose Stir the cellulose-based cellulose for 25 minutes to form a uniform insoluble paste, and then stir it with an equal amount of room temperature water for 25 minutes to completely dissolve it; add 600 mL of palmatine powder and ethyl paraben to normal saline, heat to dissolve, and Heat suction filtration to obtain the supernatant. Stir the hydroxypropyl methylcellulose solution and the palmatine supernatant evenly, add physiological saline to make the volume to 1000mL, stir well, adjust the pH value to 5.5 with an approp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com