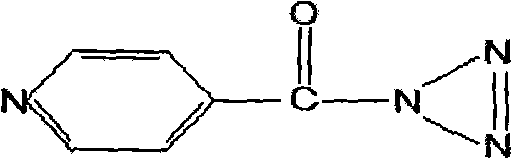
Synthesizing method of isonicotinyl hydrazine azide
A technology of azide and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of unsuitable batch production, complicated operation, high cost, etc., and achieve the effect of reducing synthesis cost, simple operation process, and simple and clear process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A synthetic method of isonicotinoyl azide, comprising the steps in turn:
[0027] (1) In the reaction kettle, according to the ratio of isoniazid moles: HCl moles=1.0:2.5, add 0.1 moles of isoniazid and 35 milliliters of hydrochloric acid aqueous solution, stir at 0°C until dissolved, the mass of hydrochloric acid aqueous solution The percentage concentration is 26%.
[0028] (2) Add 6.9 grams of sodium nitrite aqueous solution dropwise according to the ratio of moles of isoniazid:moles of sodium nitrite=1.0:1.0 under stirring, and keep the reaction temperature at 0°C-5.0°C. After the dropwise addition, the temperature was still kept at 0°C to 5°C, and the reaction was stirred for another 2h.
[0029] (3) Slowly add an aqueous solution of sodium carbonate with a concentration of 10% by mass to the reaction system under stirring until pH=7, a large amount of white crystals are precipitated from the reaction solution, filtered, and dried with concentrated sulfuric acid t...
Embodiment 2
[0031] A synthetic method of isonicotinoyl azide, comprising the steps in turn:
[0032] (1) In the reactor, add 1 mole of isoniazid and 425 milliliters of hydrochloric acid aqueous solution according to the ratio of isoniazid moles: HCl moles=1.0:3.5, stir at 0°C until dissolved, the mass of hydrochloric acid aqueous solution The percentage concentration is 30%.
[0033] (2) Add dropwise the sodium nitrite aqueous solution of 103 grams according to isoniazid molar number: the ratio of sodium nitrite molar number=1.0:1.5 under stirring, and keep reaction temperature at 0 ℃~5.0 ℃, after dropwise addition, Still keeping the temperature at 0°C to 5°C, the reaction was stirred for another 1h.
[0034] (3) Slowly add an aqueous solution of sodium carbonate with a mass percentage concentration of 15% to the reaction system under stirring until pH = 7, a large amount of white crystals are precipitated from the reaction solution, filtered, and dried with concentrated sulfuric acid to...
Embodiment 3
[0036] A synthetic method of isonicotinoyl azide, comprising the steps in turn:
[0037] (1) In the reactor, add 1.5 moles of isoniazid and 586 milliliters of hydrochloric acid aqueous solution according to the ratio of moles of isoniazid: moles of HCl=1.0:3.0, and stir until dissolved at 0° C., the mass of aqueous hydrochloric acid The percentage concentration is 28%.
[0038](2) Add dropwise the sodium nitrite aqueous solution of 124 grams according to isoniazid molar number: the ratio of sodium nitrite molar number=1.0:1.2 under stirring, and keep reaction temperature at 0 ℃~2 ℃, after dropwise addition, Still keeping the temperature at 0°C to 2°C, the reaction was stirred for another 1.5h.
[0039] (3) Slowly add an aqueous solution of sodium carbonate with a mass percentage concentration of 17.2% to the reaction system under stirring until pH = 7, a large amount of white crystals are precipitated from the reaction solution, filtered, and dried with concentrated sulfuric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com